Abstract
Purpose: To assess the efficacy and toxicity of menogaril against non-Hodgkin's lymphoma (NHL) in a group of previously treated patients.
Patients and methods: Sixty-two eligible patients with a histologic diagnosis of NHL were enrolled, 35 of who had intermediate or high-grade histologies and 27 of who had low-grade lymphomas. Patients with intermediate or high-grade lymphomas had received only 1 prior chemotherapy regimen, while patients with low-grade histologies had received 1 or 2 prior chemotherapy regimens. Menogaril was administered at 160 mg/m2 intravenously over 1 hour, once every 28 days.
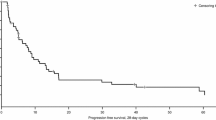
Results: Among the 35 patients with intermediate or high-grade lymphomas who were evaluable for response, 6 of 35 patients achieved a partial response (PR) for a response rate of 17% (95% confidence interval: 7%−34%). Median survival in this group of patients was 13 months. For those patients with low-grade lymphoma, 5 of 26 patients achieved a PR for a response rate of 19% (95% confidence interval: 6%−38%). No complete responses were observed in either patient group. The incidence of serious (grade 3 or 4) toxicity for those with intermediate/high-grade and low-grade lymphomas was 43% and 44%, respectively. Most of these toxic effects consisted of reversible myelosuppression. Menogaril was discontinued in 2 patients due to prolonged neutropenia. Cardiotoxicity was observed in 4 patients, requiring discontinuation of the drug in 1 patient. No treatment-related deaths occurred and the overall toxicity was felt to be acceptable.
Conclusion: The observed antitumor activity of single agent menogaril against both intermediate/high-grade and low-grade lymphomas was modest. Further exploration of this agent in patients with non-Hodgkin's lymphomas does not seem warranted.
Similar content being viewed by others
References
Clinical Brochure: Menogaril (NSC 269148). National Cancer Institute, Bethesda, MD, 1983
McGovern J, Neil G, Denlinger R, Hall T, Crampton S, Swenberg J: Chronic cardiotoxicity studies in rabbits with 7-con-O-methylnogarol, a new anthracycline antitumor agent. Cancer Res 39: 4849–4855, 1979
Brown TD, Weiss G, Kuhn J, VonHoff D, Earhart R: Orally administered menogaril (M): A phase I clinical and pharmacologic study. Proc Am Soc Clin Oncol 1987 (abstr C-136)
Dorr A, VonHoff D, Kuhn J, Schwartz R, Kisner D: Phase I clinical investigation of 7-con-methylnogarol, a new anthracycline antibiotic. Cancer Res 46: 2562–2565, 1986
Miller A, Hoogstraten B, Staquet M, Winkler A: Reporting results of cancer treatment. Cancer 47: 207–214, 1981
Skillings J, Cripps C, Eisenhauer E, Pater J, Verma S, Walde D: A phase II study of menogaril in low-grade non-Hodgkin's lymphoma. Invest New Drugs 9: 79–82, 1991
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moore, D.F., Brown, T.D., LeBlanc, M. et al. Phase II Trial of Menogaril in Non-Hodgkin's Lymphomas: a Southwest Oncology Group Trial. Invest New Drugs 17, 169–172 (1999). https://doi.org/10.1023/A:1006375301205
Issue Date:
DOI: https://doi.org/10.1023/A:1006375301205