Abstract
Although UFT 300 mg/m2/day and leucovorin 90 mg/day administered orally in divided doses administered every 8 hours for 28 days repeated every 35 days could be administered safely to patients with advanced hepatomas and good performance status, this combination and schedule has limited activity in treating advanced hepatoma.
Background/purpose: Biochemical modulation of 5-fluorouracil has yielded higher response rates in hepatoma when compared to treatment with 5-fluorouracil as a single agent, although the impact on survival has been negligible. This study was conducted to determine the activity and evaluate the toxicity of uracil and tegafur in a 4:1 molar concentration ratio (UFT; Bristol-Myers Squibb, Wallingford, CT) plus oral calcium leucovorin in the treatment of patients with advanced hepatocellular carcinoma (hepatoma).
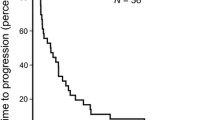
Patients and methods: Sixteen patients with advanced measurable hepatocellular carcinoma were enrolled onto the trial. All patients had a Karnofski performance status ≥ 60%, platelet count ≥ 75,000/μL, total bilirubin ≤ 2.0× institutional upper limit of normal but otherwise normal liver and kidney function profile and bidimensionally measurable disease by CT or ultrasound examination. None of these patients received prior cytotoxic chemotherapy or radiation therapy for advanced disease. Fourteen patients received 300 mg/m2/d UFT plus 90 mg/d leucovorin administered orally in divided daily doses every 8 hours for 28 days repeated every 35 days. Two patients registered for the trial but did not receive study medication. Objective tumor response, the primary purpose of this trial, was evaluated after two courses of therapy. Other end-points included toxicity, time to progression, and overall survival.
Results: Fourteen patients were evaluable for response and toxicity, respectively. No complete or partial responders were observed in this trial. Three patients had stable disease lasting 17 to 22 weeks. Toxicity was mild with severe (grade 3 or 4) liver pain, diarrhea, anorexia/nausea, fatigue, dyspnea, hyperbilirubinemia, anemia, and edema seen in 3 (21%), 2 (14%), 3 (21%), 2 (14%), 1 (7%), 1 (7%), 1 (7%) and 1(7%) patients, respectively. The most frequent grade 1 and 2 toxic effects included fever of unknown origin, dyspnea, nausea, vomiting and diarrhea.
Conclusion: UFT 300 mg/m2/d plus oral leucovorin 90 mg/d administered for 28 days did not demonstrate antitumor activity against advanced hepatomas. Further treatment using this regimen is not recommended for this disease.
Similar content being viewed by others
References
Venook AP: Treatment of hepatocellular carcinoma: too many options? J Clin Oncol 12: 1323-1334, 1994
Patt YZ, Charnsangavej G, Lawrence D et al.: Hepatic Arterial infusion (HAI) of Fluorouracil (F), Leucovorin (L), Adriamycin (A), and Platinol (P) (FLAP): effective palliation for nonresectable Hepatocellular Cancer (HCC). Proc Am Soc Clin Oncol (abstr) 11: 165, 1992
Maehara Y, Kakeji Y, Ohno S et al.: Scientific basis for the combination of Tegafur with uracil. Oncol (Suppl 10) 11: 14- 16, 1997
Pazdur R: Phase I and pharmacokinetic evaluations of UFT plus oral leucovorin. Oncol (Suppl 10) 11: 35-38, 1997
Taguchi T, Nakano H, Jikuya H et al.: Effect of uracil on the antitumor activity of Tegafur. Jpn J Cancer Chemother 5: 1161-1165, 1978
Taguchi T, Koyanna Y et al.: Phase I study of UFT. Jpn J Cancer Chemother 7: 966-972, 1980
Nakajima O, Ihara K, Isoda T et al.: Phase I and II studies on mixture of 1-(2-tetrahydrofluorouracil)-5-fluorouracil and uracil. Jpn J Cancer Chemother 7: 1558-1568, 1980
Taguchi T: Experience with UFT in Japan. (Suppl 10) Oncol 11: 30-35, 1997
Ota K, Taguchi T, Kimura K: Report on nationwide pooled data and cohort investigation in UFT phase II study. Cancer Chemother Pharmacol 22: 333-338, 1988
Tokyo Liver Cancer Chemotherapy Study Group: Phase II study of co-administration of Uracil and Tegafur (UFT) in hepatocellular carcinoma. Jpn J Clin Oncol 15: 559-562, 1985
Mani S: An ongoing phase II study of UFT plus leucovorin in advanced hepatobiliary tumors and pancreatic adenocarcinomas. Oncol (Suppl 10) 11: 124-128, 1997
Ajani JA, Welch SR, Raber MN et al.: Comprehensive criteria for survival in therapy-induced toxicity. Cancer Invest 8: 141-153, 1990
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mani, S., Schiano, T., Garcia, J.C. et al. Phase II trial of uracil/tegafur (UFT) plus leucovorin in patients with advanced hepatocellular carcinoma. Invest New Drugs 16, 279–283 (1998). https://doi.org/10.1023/A:1006104217137
Issue Date:
DOI: https://doi.org/10.1023/A:1006104217137