Abstract
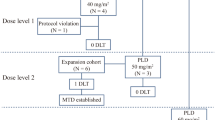
Purpose: Edatrexate (10-Edam) is a methotrexate analog with improved intracellular transport, polyglutamation, and antitumor activity compared to the parent compound. Edatrexate shows schedule-dependent synergism with platinum compounds in preclinical studies. We performed a phase I trial to determine toxicities and establish the maximum tolerated dose (MTD) of edatrexate in combination with carboplatin. Based on the short initial plasma half-life of edatrexate, prophylactic ice chip cryotherapy was used to reduce the severity of mucositis. Patients and methods: Forty-six chemotherapy-naive patients with advanced solid tumors were treated. Edatrexate was given weekly for 5 doses (50% on day 8), and then every other week, followed by carboplatin at a fixed dose of 350 mg/m2 on day 1 and then every 4 weeks for 8 cycles. Edatrexate dose was increased at increments of 10 mg/m2/dose level beginning at 60 mg/m2/week (range 60–120 mg/m2). Results: All patients were assessable for toxicity and response analysis. The median number of cycles administered per patients was 4. This combination chemotherapy regimen was well tolerated. Using ice chip cryotherapy, no grade IV mucositis was observed. Grade III mucositis occurred in only 7/46 pts and was not dose-related. Protocol-defined dose-limiting toxicity occurred at a edatrexate dose level of 120 mg/m2, yielding an MTD of 110 mg/m2. Responding tumor types included non-small cell and small lung cancer, head and neck cancer, and bladder cancer. Conclusions: 1) This phase I study demonstrated the safety and tolerability of this edatrexate and carboplatin combination. 2) Dose-limiting mucositis did not occur allowing escalation of edatrexate dose above levels previously achieved with this edatrexate dose schedule. This was most likely a result of prophylactic ice chip cryotherapy. 3) An edatrexate dose of 110 mg/m2 with ice chip cryotherapy is recommended for Phase II trials of this combination.
Similar content being viewed by others
References
Sirotnak FM, DeGraw JI, Moccio DM, Samuels LL, Goutas LJ: New folate analogs of the 10-deaza-aminopterin series. Basis for structural design and biochemical and pharmacological properties. Cancer Chemother Pharmacol 12:18-25, 1984
Schmid FA, Sirotnak FM, Otter GM, DeGraw JI: New folate analogs of the 10-deaza-aminopterin series. Markedly increased antitumor activity of the 10-ethyl analog compared to the parent compound and methotrexate against some human tumor xenografts in nude mice. Cancer Treat Rep 69:551-552, 1985
Sirotnak FM, DeGraw JI, Schmid FA, Goutas LJ, Moccio DM: New folate analogs of the 10-deaza-aminopterin series. Further evidence for markedly increased antitumor efficacy compared with methotrexate in ascitic and solid murine tumor models. Cancer Chemother Pharmacol 12:26-30, 1984
Canetta R, Goodlow J, Smaldone L, Bragman K, Rozencweig M: Pharmacologic characteristics of carboplatin: clinical experience. In: Bunn Jr PA, Canetta R, Ozols RF, Rozencweig M (eds) Carboplatin (JM-8). Current Perspective and Future Directions. Philadelphia: WB Saunders Company, pp 19-38, 1990
Chou TC, Tan QH, Sirotnak FM: Quantitation of the synergistic interaction of edatrexate and cisplatin in vitro. Cancer Chemother Pharmacol 31:259-264, 1993
Perez EA, Hack FM, Webber L, Chou TC: Schedule dependent synergism of edatrexate and cisplatin combination in the A549 lung cancer cell line as assessed by median effect analysis. Cancer Chemother Pharmacol 33:245-250, 1993
Perez EA, Hack FM, Fletcher T, Chou TC: Modulation of intrinsic in vitroresistance to carboplatin by edatrexate. Oncol Res 34:331-334, 1994
Lee JS, Libshitz HI, Murphy WK, Jeffries D, Hong WK: Phase II study of 10-ethyl-10-deaza-aminopterin (10-EdAM; CGP 30 694) for stage III B or IV non-small cell lung cancer. Invest New Drugs 8:299-304, 1990
Lee JS, Murphy WK, Shirinian MH, Pang A, Hong WK: Alleviation by leucovorin of the dose-limiting toxicity of edatrexate: potential for improved therapeutic efficacy. Cancer Chemother Pharmacol 28:199-204, 1991
VandenBerg RA, Pritchard KI, Eisenhauer EA, Trudeau ME, Norris BD, Lopez P, Verma SS, Buckman RA, Muldal A: Phase II study of weekly edatrexate as first-line chemotherapy for metastatic breast cancer: a National Cancer Institute of Canada Trials Group Study. J Clin Oncol 11:1241-1244, 1993
Schornagel JH, Verweij J, de Mulder PHM, Cognetti F, Vermorken JB, Cappaelaere P, Armand JP, Wildiers J, Clavel M, Sahmoud T, Kirkpatrick A, Lefebvre JL: Randomized phase III trial of edatrexate versusmethotrexate in patients with metastatic and/or recurrent squamous cell carcinoma of the head and neck: a European Organization for Research and Treatment of Cancer Head and Neck Center Cooperative Group Study. J Clin Oncol 13:1649-1655, 1995
Shum KY, Kris MG, Gralla RJ, Burke T, Marks LD, Heelan RT: Phase II study of 10-ethyl-10-deaza-aminopterin in patients with stage III and IV non-small cell lung cancer. J Clin Oncol 6:446-450, 1988
Kris MG, Kinahan JJ, Gralla RJ, Fanucchi MP, Wertheim MS, O'Connell JP, Marks LD, Williams L, Farag F, Young CW, Sirotnak FM: Phase I trial and clinical pharmacological evaluation of 10-ethyl-10-deaza-aminopterin in adult patients with advanced cancer. Cancer Res 48:5573-5579, 1988
Gandara DR, Perez EA, Edelman MJ, Lau D, Lauder I, Turrell C, Meyers F: Phase I trial of edatrexate plus carboplatin in advanced solid tumors: amelioration of dose-limiting toxicity mucositis by ice chip cryotherapy. Proc Am Soc Clin Oncol 14:468, 1995
Mahood DJ, Dose AM, Loprinzi CL, Veeder MH, Athmann LM, Therneau TM, Sorensen JM, Gainey DK, Mailliard JA, Gusa NL, Finck GK, Johnson C, Goldberg RM: Inhibition of fluorouracil-induced stomatitis by oral cryotherapy. J Clin Oncol 9:449-452, 1991
Hartley JM, Nicholson PW, Allen R, Lamond P, Harland SJ, Souhami RL: Pharmacokinetics of 10-ethyl-10-deazaaminopterin, edatrexate, given weekly for non-small-cell lung cancer. Cancer Chemother Pharmacol 31:328-332, 1993
Grant SC, Kris MG, Young CW, Sirotnak FM: Edatrexate, an antifolate with antitumor activity: a review. Cancer Invest 11(1):36-45, 1993
Lee JS, Libshitz HI, Fosella FV, Murphy WK, Pang AC, Lippman SM, Shin DM, Dimery IW, Glisson BS, Hong WK: Edatrexate improves the antitumor effects of cyclophosphamide and cisplatin against non-small cell lung cancer. Cancer 68:959-964, 1991
Grunberg SM, Spears CP, Natale R, Zaretsky S, Ashikaga T: Phase I evaluation of high-dose edatrexate (EDX) with leucovorin (LV) rescue. ProcAm Soc Clin Oncol 13:146, 1994
Fosella FV, Lee JS, Winn R, Wester M, Graham S, Holder LW, Goodwin JW, Dakhil SR, Hong WK: Edatrexae (E) + Ifosfamide (I) + cisplatin (P) with leucovorin (LV) and mesna (M) for advanced non-small cell lung cancer (NSCLC). Proc Am Soc Clin Oncol 14:371, 1995
Perez EA, Whitall D, Hesketh PJ: Phase I trial of biweekly edatrexate in metastatic breast cancer. Proc Am Soc Clin Oncol 13:59, 1994
Sirotnak FM, Otter GM, Schmid FA: Markedly improved efficacy of edatrexate compared to methotrexate in a highdose regimen with leucovorin rescue against metastatic murine solid tumors. Cancer Res 53(3):587-591, 1993
Jolivet J, Jansen G, Peters GJ, Pinard MF, Schornagel JH: Leucovorin rescue of human cancer and bone marrow cells following edatrexate or methotrexate. Biochem Pharmacol 47(4):659-665, 1994
Calvert AH, Newell DR, Gumbrell LA, O'Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME, Wiltshaw E: Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 7:1748-1756, 1989
Izquierdo MA, Sanchez A, Llort G, Moreno V, Rey M, Germa JR: Comparison of different methods for AUC dosing of carboplatin (cbdca). Proc Am Soc Clin Oncol 16:204a(#714), 1997
Paesmans M, Sculier JP, Thiriaux J, Bureau G, Giner V, Efremidis A, Lafitte JJ, Berchier MC, Alexopoulos CG, Zacharias C, Mommen P, Ninane V, Klastersky J: Carboplatin prescribed in mg/m2 in patients with advanced non-small cell lung cancer (nsclc): is there an impact of the reached area under the curve (auc) on response, survival and hematological toxicity? Proc Am Soc Clin Oncol 16:205a(#716), 1997
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Edelman, M.J., Gandara, D.R., Perez, E.A. et al. Phase I trial of edatrexate plus carboplatin in advanced solid tumors: amelioration of dose-limiting mucositis by ice chip cryotherapy. Invest New Drugs 16, 69–75 (1998). https://doi.org/10.1023/A:1006026928733
Issue Date:
DOI: https://doi.org/10.1023/A:1006026928733