Abstract
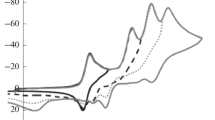
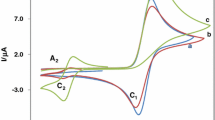
Electrochemical oxidation potentials of 1,4-dihydropyridines substituted with 4-COOH, 4-COOR, and 4-CONRR' groups have been determined in aprotic acetonitrile by the rotating ring-disk electrode method (RRDE). The electrochemical reduction potentials of the resulting products were also determined at the ring electrode. It was established that protonated pyridines are formed in the oxidation of derivatives with and without a substituent in position 4 of the heterocycle. In the case of 4-alkoxycarbonyl substituted compounds the substituent at position 4 is generally retained. 1,4-Dihydrogenated derivatives of isonicotinic acid as a rule loose the substituent at position 4 on oxidation, and both types of product were recorded for the corresponding 4-carbamoyl derivatives. The substituent at position 9 of the heterocycle was mainly retained on electrochemical oxidation of the 3,3,6,6-tetramethyl-1,8-dioxo-1,2,3,4,5,6,7,8,9,10-decahydroacridine derivatives studi! ed.
Similar content being viewed by others
REFERENCES
P. J. Elving, C. O. Schmakel, and K. S. V. Santhanam, Crit. Rev. Anal. Chem., 6, 1 (1976).
I. Misane, V. Klusa, M. Dambrova, S. Germane, G. Duburs, E. Bisenieks, R. Rimondini, and S. O. Ögren, Europ. Neuropsychopharmacology, 8, 329 (1998).
M. B. Taraban, A. I. Krupa, N. E. Polyakov, T. V. Leshina. V. Lusis, D. Muceniece, and G. Duburs, J. Photochem. Photobiol. A: Chem., 73, 151 (1993).
R. H. Böcker and F. P. Guengerich, J. Med. Chem., 29, 1596 (1986).
O. Garcia, F. Delgado, A. C. Cano, and C. Alvarez, Tetrahedron Lett., 34, 623 (1993).
O. Augusto, H. S. Beilan, and P. R. Ortiz de Montellano, J. Biol. Chem., 257, 11288 (1982).
Ya. P. Stradyn', Yu. I. Beilis, Ya. R. Uldrikis, G. Ya. Dubur, A. E. Sausin', and B. S. Chekavichus, Khim. Geterotsikl. Soedin., 1525 (1975).
J. Ogle, J. Stradins, and L. Baumane, Electrochim. Acta, 39,No. 1, 73 (1994).
V. Skala, J. Volke, V. Ohanka, and J. Kuthan, Collect. Czech. Chem. Commun., 42, 292 (1977).
J. Ludvik, F. Turecek, and J. Volke, J. Electroanal. Chem., 188, 105 (1985).
G. Ya. Dubur and Ya. R. Uldrikis, Khim. Geterotsikl. Soedin., 354 (1972).
G. Ya. Dubur and G. Ya. Vanag, Izv. Akad. Nauk LatvSSR, Ser. Khim., 119 (1962).
R. W. Saalfrank, S. Reihs, and M. Hug, Tetrahedron Lett., 34, 6036 (1993).
J.-J. V. Eynde, R. D'Orazio, and Y. V. Haverbeke, Tetrahedron, 50, 2479 (1994).
F. P. Guengerich, W. R. Brain, M. Iwasaki, M.-A. Sari, C. Bäärnhielm, and P. Berntsson, J. Med. Chem., 34, 1838 (1991).
N. R. Konopleva, Ya. P. Stradyn', N. P. Skvortsov, G. Ya. Dubur, and Yu. V. Vodzinskii, Izv. Akad. Nauk LatvSSR, Ser. Khim., 678 (1983).
G. Ya. Dubur and Ya. R. Uldrikis, Khim. Geterotsikl. Soedin., 1015 (1969).
J. Poikans, G. Tirzitis, E. Bisenieks, J. Uldrikis, V. S. Gurevich, I. A. Mikhailova, and G. Duburs, Eur. J. Med. Chem., 29, 325 (1994).
A. Kalnins, L. Baumane, J. Stradins, E. Bisenieks, J. Poikans, J. Uldrikis, and G. Duburs, Latv. Kim. Zh., No. 4, 71 (1998).
J. F. Biellmann and H. J. Callot, Tetrahedron, 26, 4799 (1970).
R. J. Highet and J. F. Biellmann, J. Org. Chem., 5, 37 (1972).
M. F. Bundule, E. A. Bisenieks, A. A. Kemme, Ya. Ya. Bleidelis, G. Ya. Dubur, and Ya. R. Uldrikis, Khim. Geterotsikl. Soedin., 666 (1980).
Ya. R. Uldrikis, G. Ya. Dubur, I. V. Dipan, and B. S. Chekavichus, Khim. Geterotsikl. Soedin., 1230 (1975).
A. E. Sausin', V. K. Lusis, G. Ya. Dubur, and Yu. I. Beilis, Khim. Geterotsikl. Soedin., 1508 (1978).
M. R. Tarasevich, E. I. Khrushcheva, and V. Yu. Filinovskii, The Rotating Disk Electrode with a Ring [in Russian], Nauka, Moscow (1987), p. 247.
D. Clark, M. Fleishmann, and D. Pletcher, J. Electroanal. Chem., 36, 137 (1972).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stradins, J., Baumane, L., Kalnins, A. et al. Special Features of the Electrochemical Oxidation of Substituted 4-Carboxy-1,4-dihydropyridines. Chemistry of Heterocyclic Compounds 36, 1177–1184 (2000). https://doi.org/10.1023/A:1002868600011
Issue Date:
DOI: https://doi.org/10.1023/A:1002868600011