Abstract
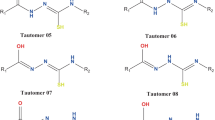
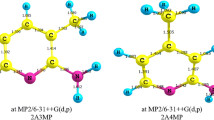
The AM1 quantum-chemical semi-empirical method was used to calculate the values of the enthalpy of activation (ΔΔH#), the heats of reaction (ΔΔH), and the tautomeric equilibrium constants (KT) for several α-substituted pyridines. It was found that the keto-enol tautomeric conversion in α-pyridone occurs more readily than the amino-imino conversion in 2-aminopyridine but in 2-methylpyridine the tautomeric equilibrium does not exist at all.
Similar content being viewed by others
REFERENCES
A. R. Katritzky and J. M. Lagowski, Adv. Heterocycl. Chem., 1, 339 (1963).
J. Frank and A. R. Katritzky, J. Chem. Soc., Perkin. Trans. 2, 1428 (1976).
M. J. S. Dewar and G. J. Gleicher, J. Amer. Chem. Soc., 87, 685 (1965).
J. A. Kereselidze, Khim. Geterotsikl. Soedin., 752 (1999).
K. Inuzuka and A. Fujimoto, Spectrochim. Acta, 40A,7, 623 (1984).
A. R. Katritzky and J. M. Lagowski, The Chemistry of Heterocyclic Compounds [Russian translation], Inostr. Lit., Moscow (1963).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kereselidze, J.A., Zarkua, T.S. Features of the Tautomerism of Some α-Substituted Pyridines. Chemistry of Heterocyclic Compounds 36, 1161–1163 (2000). https://doi.org/10.1023/A:1002860430962
Issue Date:
DOI: https://doi.org/10.1023/A:1002860430962