Abstract
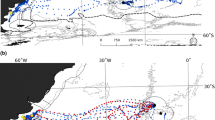
Southern sea lions (Otaria flavescens; SSLs) have a polygynous mating system and a prolonged social-sexual maturation period for males. Male haulouts are common in this species, with some very distant from central breeding rookeries, although the functions of these aggregations are not clearly understood. To estimate the potential connectivity between northern Argentina male colonies and breeding rookeries in Patagonia and Uruguay, we monitored the summer breeding activities and winter presence of 559 individually identified SSL males from haulouts in Mar del Plata and Quequén harbors. Our results confirm that male groups are formed by sexually active sea lions that show a strong annual connection with distant (up to 700 km) breeding colonies. Circa 70% of the marked males made long-distance round-trips (72 ±26.7 days; n = 325) from northern Argentina to Patagonia and Uruguay, indicating a high degree of winter site fidelity. Mating activity was confirmed for 53% of the sea lions re-sighted in breeding colonies, with approximately 80% of them having central positions on the beach and holding harems of up to nine females. The chronology of this cycle is finely tuned with the onset of the breeding season, which may result in comparative advantages such as anticipating female arrival or a prolonged participation in mating activities. Our results suggest a model of male haulouts spatially segregated from the central breeding areas, but with a summer recurrent flow of animals that contributes a significant proportion of the male population of northern Patagonia and Uruguay.
Similar content being viewed by others

References
Agresti, A., 1990. Categorical Data Analysis. John Wiley and Sons, New York.
Atkinson, S., 1997. Reproductive biology of seals. Rev. Reprod. 2, 175–194.
Aurioles, D., Sinsel, F., Fox, C, Alvarado, E., Maravilla, O., 1983. Winter migration of subadult male California sea lions (Zalophus californianus) in the southern part of Baja California. J. Mamm. 64, 513–518.
Aurioles-Gamboa, D., Elorriaga-Verplancken, F., Hernández-Camacho, C.J., 2010. The current population status of Guadalupe fur seal (Arctocephalus townsendi) on the San Benito Islands. Mexico. Mar. Mamm. Sci. 26, 402–408.
Bartholomew, G.A., 1970. A model forthe evolution of pinniped polygyny. Evolution 24, 246–559.
Beentjes, M.P., 1989. Haul out patterns, site fidelity and activity budgets of male Hooker’s sea lions (Phocarctos hookeri) on the New Zealand mainland. Mar. Mamm. Sci. 5, 281–297.
Belkin,A.N., 1966. Summer distribution, stocks, prospects for commercial utilization and certain features of the biology of sea lions inhabiting the Kurile Islands. Iavestiya Tikhookeanskogo N-I (TINRO) 58, 69–95.
Bester, M.N., 1982. An analysis of the southern elephant seal Mirounga leonina breeding population at Kerguelen. S. Afr.J. Antarct. Res. 12, 11–16.
Boness, D.J., 1991. Determinants of mating systems intheOtariidae(Pinnipedia). In: D.R. (Ed.), Behaviour of Pinnipeds. Chapman & Hall, London, pp. 1–44.
Boyd, I.L., McCafferty, D.J., Reid, K., Taylor, R., Walker, T.R., 1998. Dispersal of male and female Antarctic fur seals (Arctocephalus gazella). Can. J. Fish. Aquat. Sci. 55, 845–852.
Campagna, C, 1985. The breeding cycle of the Southern sea lion Otaria byronia. Mar. Mamm. Sci. 1, 210–218.
Campagna, C, Le Beouf, B.J., 1988. Reproductive behaviour of southern sea lions. Behaviour 104, 233–261.
Campagna, C, Werner, R., Karesh, W., Marin, M.R., Koontz, F., Cook, R., Koontz, C, 2001. Movements and location at sea of South American sea lions (Otaria flavescens). J. Zool. 257, 205–220.
Carrara, I.S., 1952. Lobos marinos, pinguinos y guaneras del litoral maritimo e islas adyacentes de la Republica Argentina. Catedra de Higiene e Industrias, Facultad de Ciencias Veterinarias. Universidad Nacional de La Plata, La Plata, Argentina.
Cassini, M.H., 2000. A model on female breeding dispersion and the reproductive systems of pinnipeds. Behav. Process. 51, 93–99.
Cassini, M., Fernández-Juricic, E., 2003. Costs and benefits of joining South American sea lion breeding groups: testing the assumptions of a model of female breeding dispersion. Can. J. Zool. 81, 1154–1160.
Chilvers, B.L., Wilkinson, I.S., 2008. Philopatry and site fidelity of New Zealand sea lions (Phocarctos hookeri). Wildl. Res. 35, 463–470.
Conradt, L., 1998. Could asynchrony in activity between the sexes cause intersexual social segregation in ruminants? Proc. R. Soc. Lond. B 265, 1359–1368.
Conradt, L, 2005. Definitions, hypotheses, models and measures in the study of animal segregation. In: Ruckstuhl, K.E.N.P. (Ed.), Sexual Segregation in Vertebrates. Ecology of the Two Sexes. Cambridge University Press, Cambridge.
Crespo, E.A., (PhD thesis) 1988. Dinámica poblacional del lobomarinodel sur Otaria flavescens (Shaw 1800) en el norte del litoral patagonico. Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Buenos Aires, Argentina, pp. 298.
Crooks, K.R., Sanjayan, M., 2006. Connectivity Conservation. Cambridge University Press, Cambridge, UK.
Dans, S.L., Crespo, E.A., Pedraza, S.N., Koen Alonso, M., 2004. Recovery of the South American sea lion (Otaria flavescens) population in northern Patagonia. Can. J. Fish. Aquat. Sci. 61, 1681–1690.
Dobson, S.F., 1982. Competition for mates and predominant juvenile male dispersal in mammals. Anim. Behav. 30, 1183–1192.
Drago, M., Cardona, L., Aguilar, A., Crespo, E.A., Ameghino, S., García, N., 2010. Diet of lactating South American sea lions, as inferred from stable isotopes, influences pup growth. Mar. Mamm. Sci. 26, 309–323.
Erickson, A.W., Bester, M.N., Laws, R.M., 1993. Marking techniques. In: Laws, R.M. (Ed.), Antarctic Seals. Research Methods and Techniques. Cambridge University Press, Cambridge, UK, pp. 89–118.
Feijoo, M., Lessa, E.P., Loizaga de Castro, R., Crespo, E.A., 2011. Mitochondrial and microsatellite assessment of population structure of South American sea lion (Otaria flavescens) in the Southwestern Atlantic Ocean. Mar. Biol. 158, 1857–1867.
Franco-Trecu, V., Aurioles-Gamboa, D., Arim, M., Lima, M., 2012. Prepartum and postpartum trophic segregation between sympatrically breeding female Arctocephalus australis and Otaria flavescens. J. Mamm. 93, 514–521.
Frankham, R., Ballou, J.D., Briscoe, D.A., 2002. Introduction to Conservation Genetics. Cambridge University Press.
Gentry, R.L., Holt, J.R., 1982. Equipment and techniques for handling northern fur seals. NOAATech. Rep. NMFS, 16.
Giardino, G.V., (BSc (Hons.) thesis) 2006. Patrones de ingreso y egreso y estimación del tamaño de la colonia de lobos marinos de un pelo de Puerto Quequén. Facultad de Ciencias Exactas y Naturales Departamento de Ciencias Marinas. Universidad Nacional de Mar del Plata, pp. 67.
Giardino, G., Mandiola, A., Bastida, J., Bastida, R., Rodríguez, D., 2013. Técnica de marcado pordecoloraciónde pelo en el lobo marino Otaria flavescens: descripcióny evaluación del método. Mastozool. Neotrop. 20, 393–398.
Giardino, G., (PhD thesis) 2014. Estructura y dinámica de las colonias de lobos marinos de un pelo de la Provincia de Buenos Aires, y su relación con pesquerías de la región. Departamento de Ciencias Marinas, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Mar del Plata, Mar del Plata, pp. 173.
Grandi, M.F., Dans, S.L., Crespo, E.A., 2008. Social composition and spatial distribution of colonies in an expanding population of South American sea lions. J. Mamm. 89, 1218–1228.
Grandi, M.F., Dans, S.L., García, N.A., Crespo, E.A., 2010. Growth and age at sexual maturity of South American sea lions. Mamm. Biol. 75, 427–436.
Grandi, M.F., Oliveira, D.L.R., Dans, S.L., Crespo, E.A., 2012. A hunted population in recovery: effective population size for South American sea lions from Patagonia. Anim. Biol. 62, 433–450.
Greenwood, P.J., 1980. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28, 1140–1162.
Hamilton, J.E., 1934. The southern sea lion, Otaria byronia. Dis. Rep. VIII, 269–318.
Hamilton, J.E., 1939. A second report on the southern sea lion, Otaria byronia de Blainville. Dis. Rep. 19, 121–164.
Hill, T., Lewicki, P., 2007. STATISTICS Methods and Applications. Statsoft, Tulsa, Oklahoma.
Hoffman, J.I., Trathan, P.N., Amos, W., 2006. Genetic tagging reveals extreme site fidelity in territorial male Antarctic fur seals Arctocephalus gazella. Mol. Ecol. 15, 3841–3847.
Jarnemo, A., 2008. Seasonal migration of male red deer (Cervus elaphus) in southern Sweden and consequences for management. Eur. J. Wildl. Res. 54, 327–333.
Koen Alonso, M., Crespo, E.A., Pedraza, S.N., Gacía, N.A., Coscarella, M.A., 2000. Food habits of the South American sea lion, Otaria flavescens, off Patagonia, Argentina. Fish. Bull. 98, 250–263.
LeBoeuf, B.J., 1991. Pinniped mating systems on land, ice and in the water: emphasis on the Phocidae. In: Reese, E.S., Lighter, F.J. (Ed.), Behavior of Pinnipeds. John Wiley and Sons, New York, pp. 251–279.
Lewis, M., Ximenez, I., 1983. Dinámica de la población de Otaria flavescens en el área de Península Valdés zonas adyacentes (Segunda parte), Contribución No. 79. Centro Nacional Patagónico, pp. 21.
Lindenfors, P., Tullberg, B., Biuw, M., 2002. Phylogenetic analyses of sexual selection and sexual size dimorphism in pinnipeds. Behav. Ecol. Sociobiol. 52, 188–193.
Lockwood, D.R., Hastings, A., Botsford, L.W., 2002. The effects of dispersal patterns on marine reserves: does the tail wag the dog? Theor. Popul. Biol. 61, 297–309.
Lorenzani, J., Lorenzani, J., 1992. Resultados preliminares de marcación de de lobos marinos de un pelo Otaria flavescens de la colonia del puerto de Mar del Plata. 5ta Reunión de especialistas en Mamíferos acuáticos de América del Sur, Buenos Aires-Argentina, pp. 41.
Lorenzani, J., Lorenzani, J., 1998. Resultados preliminares de marcación de lobos marinos de un pelo (Otaria flavescens) en el puerto de Quequén, Pcia de Bs As. In: 8 ° Reunião de Trabalho de Especialistas em Mamíferos Aquáticos da América do Sul (RT) 2° Congresso da Sociedade Latinoamericana de Especialistas em Mamíferos Aquáticos (SOLAMAC), Olinda-PE-Brasil, p. 115.
Lowther, A.D., Harcourt, R.G., Hamer, D.J., Goldsworth, S.D., 2011. Creatures of habit: foraging habitat fidelity of adult female Australian sea lions. Mar. Ecol. Prog. Ser. 443, 249–263.
Lowther, A.D., Harcourt, R.G., Goldsworthy, S.D., Stow, A., 2012. Population structure of adult female Australian sea lions is driven by fine-scale foraging site fidelity. Anim. Behav. 83, 691–701.
Main, M.B., Weckerly, F.W., Vernon, C.B., 1996. Sexual segregation in ungulates: new directions for research. J. Mamm. 77, 449–461.
Mandiola, A., (BSc (Hons.) thesis) 2009. Patrones de utilización de sectores internos del Puerto de Mar del Plata por parte de lobos marinos de un pelo. Facultad de Ciencias Exactas y Naturales, Departamento de Ciencias Marinas. Universidad Nacional de Mar del Plata, pp. 117.
Maravilla-Chávez, M.O., Zavala-González, A., Ortega-Rubio, A., 2006. Four seasons abundance changes of Zalophus californianus californianus (Lesson 1828), Allen, 1880, in the Gulf of California, Mexico. Braz. Arch. Biol. Technol. 49, 111–116.
McConkey, S., Heinrich, S., Lalas, C, McConnell, H., McNally, N., 2002. Pattern of immigration of New Zealand sea lions Phocarctos hookeri to Otago, New Zealand: implications for management. Aust. Mamm. 24, 107–116.
Müller, G., (PhD thesis) 2004. The foraging ecology of South American sea lions (Otaria flavescens) onthe Patagonian shelf. Christian-Albrechts-Universität, Kiel, Germany, pp. 138pp.
Perlov, A.S., 1980. Age composition and sex ratio in Steller sea lions, Eumetopias jubatus (Otariidae) on Kurile Island rookeries. Zool. Zhur. 49, 1545–1553.
Ponce de León, A., Pin, O., 2006. Distribución, reproducción y alimentación del lobo fino Arctocephalus australis y del león marino Otaria flavescens en Uruguay. In: Menafra, R., Rodríguez-gallego, L, Scarabino, F., Conde, D. (Ed.), Bases para la conservación y el manejo de la costa uruguaya. VIDA SILVESTRE URUGUAY, Montevideo, pp. 305–313.
Raum Suryan, L.K., Pitcher, K.W., Calkins, D.G., Sease, J.L, Loughlin, T.R., 2002. Dispersal, rookery fidelity, and metapopulation structure of Steller sea lions (Eumetopias jubatus) in an increasing and decreasing population in Alaska. Mar. Mamm. Sci. 18, 746–764.
Riedman, M., 1990. The Pinnipeds: Seals, Sea Lions and Walruses, 1st ed. University of California Press.
Robertson, B.C., Chilvers, B.L., Duignan, P.J., Wilkinson, I.S., Gemmel, N.J., 2006. Dispersal of breeding, adult male Phocarctos hookerri: implications for disease transmission, population management and species recovery. Biol. Conserv. 127, 227–236.
Rodríguez, D.H., (BSc (Hons.) thesis) 1990. Aspectos biológicos, ecológicos e históricos de la colonia de lobos marinos de un pelo, Otaria flavescens (Shaw, 1800), el Puerto Mar del Plata. Facultad de Ciencias Exactas y Naturales. Universidad Nacional de Mar del Plata (Mar del Plata, Argentina), Mar del Plata, pp. 168. Rodríguez, D.H., (PhD thesis) 1996. Biología y Ecología de los Pinnípedos del Sector Bonaerense. Facultad de Ciencias Exactas y Naturales. Universidad Nacional de Mar del Plata, Mar del Plata, Mar del Plata, pp.351.
Rodríguez, D., Bastida, R., 1998. Four hundred years in the history of pinniped colonies around Mar del Plata, Argentina. Aquat. Conserv. Mar. Freshw. Ecosys. 8, 721–735.
Rodríguez, D.H., Dassis, M., Ponce de León, A., Barreiro, C, Farenga, M., Bastida, R.O., Davis, R.W., 2013. Foraging strategies of Southern sea lion females in the La Plata River Estuary (Argentina-Uruguay). Deep-Sea Res. Pt. II 88–89, 120–130.
Rosas, F.W.C., Pinedo, M.C., Marmontel, M., Haimovici, M., 1994. Seasonal movements of the South American sea lion (Otaria flavescens Shaw) off the Rio Grande do Sul coast. Braz. Mamm. 58, 51–59.
Ruckstuhl, K.E., Neuhaus, P., 2002. Sexual segregation in ungulates: a comparative test of three hypotheses. Biol. Rev. 77, 77–96.
Ruckstuhl, K.E., Neuhaus, P., 2005. Sexual Segregation in Vertebrates: Ecology of the Two Sexes. Cambridge University Press.
Sepúlveda, M., Quiñones, R.A., Carrasco, P., José Pérez-Álvarez, M., 2012. Daily and seasonal variation in the haul-out behavior of the South American sea lion. Mamm. Biol. 77, 288–292.
Szapkievich, V.A., Cappozzo, H.L., Crespo, E.A., Bernabeu, R.O., Comas, C, Mudry, M.D., 1999. Genetics relatedness in two Southern sea lion (Otaria flavescens) rookeries in the southwestern Atlantic. Mamm. Biol. 64, 1–5.
Thompson, D., Duck, CD., McConnell, B.J., Garrett, J., 1998a. Foraging behaviour and diet of lactating female southern sea lions (Otaria flavescens) in the Falkland Islands. J. Zool. 246, 135–146.
Thompson, P.M., Mackay, A., Tollit, D.J., Enderby, S., Hammond, P.S., 1998b. The influence of body size and sex on the characteristics of harbour seal foraging trips. Can. J. Zool. 76, 1044–1053.
Túnez, J.I., Centrón, D., Cappozzo, H.L., Cassini, M.H., 2007. Geographic distribution and diversity of mitochondrial DNA haplotypes in South American sea lions (Otaria flavescens) and fur seals (Arctocephalus australis). Mamm. Biol. 72, 193–203.
Túnez, J., Cappozzo, H., Nardelli, M., Cassini, M., 2010. Population genetic structure and historical population dynamics of the South American sea lion, Otaria flavescens, in north-central Patagonia. Genetica 138, 831–841.
Vaz Ferreira, R., 1956. Caracteristicasgenerales de las islas uruguayas habitadas por lobos marinos. Trabajos sobre Islas de Lobos y Lobos Marinos (SOYP, Uruguay), pp. 23.
Vaz Ferreira, R., 1960. Islas de Lobos y lobos marinos en Uruguay. Bol. Inf. Dto. Cient. Tec. SOYP (Uruguay) 1, 19–25.
Vaz Ferreira, R., 1975. Behaviour of the southern sea lion, Otaria flavescens Shaw, in Uruguayan islands. Rapports et Proces Verbaux des Reunions du Conseil Internationel pour la Exploration de la Mer 169, 219–227.
Vaz Ferreira, R., 1981. South American sea lion, Otaria flavescens. In: Ridgway, S.H., Harrison, R.J. (Eds.), Handbook of Marine Mammals. Academic Press, London, UK.
Vaz Ferreira, R., 1982. Otaria flavescens (Shaw), South American sea lion. FAO Mammals in the Seas IV, 477–496.
Vaz Ferreira, R., Vallejo, S., Huertas, M.D., 1984. Estudios comparativos de los etogra-mas de Otaria flavescens, Arctocephalus australis y otros Otaríidos (Mammalia). Rev. Bras. Biol. 2, 171–180.
Wartzok, D., 1991. Physiology of behaviour in pinnipeds. In: Renouf, D. (Ed.), Behavior of Pinnipeds. Chapman and Hall, London, pp. 236–299.
Werner, R., Campagna, C, 1995. Diving behavior of lactating southern sea lions (Otaria flavescens) in Patagonia. Can.J. Zool. 73, 1975–1982.
Young, J.K., Hernandez-Camacho, C.J., Gerber, L.R., 2007. Long-distance movement of a pinniped neonate. Mar. Mamm. Sci. 23, 926–930.
Zar, J.H., 2007. Biostatistical Analysis, 5nd ed. Prentice-Hall Inc., Englewood Cliffs, NJ, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giardino, G.V., Mandiola, M.A., Bastida, J. et al. Travel for sex: Long-range breeding dispersal and winter haulout fidelity in southern sea lion males. Mamm Biol 81, 89–95 (2016). https://doi.org/10.1016/j.mambio.2014.12.003
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.mambio.2014.12.003