Abstract
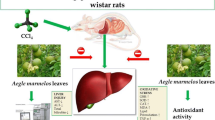
The current study explored hepatoprotective effect of Aegle marmelos(L.) Corrêa, Rutaceae, leaves extract. Potentiation of A. marmelos hepatoprotective effect with piperine co-administration was also explored. Wistar rats were randomly divided into seven groups: (i) normal control, (ii) paracetamol group, (iii) silymarin group, (iv) extract-25 group (25 mg/kg body), (v) extract-50 group: (50 mg/kg), (vi) extract-100 group (100 mg/kg) and (vii) extract-25 + piperine group. Hepatotoxicity was induced by administering paracetamol orally in a dose of 400 mg/kg for seven days. The drugs were administered 30 min prior to paracetamol administration and continued for seven days. Animals were ‘sacrificed’ at the end of treatment and serum was collected for evaluating alkaline phosphatase, bilirubin, lactate dehydrogenase, alanine aminotransferase, aspartate aminotransferase IL-10 and TNF-α levels. Liver homogenates were used for determination of oxidative stress (malondialdehyde, reduced glutathione, superoxide dismutase, catalase, glutathione reductase, GSH-S-transferase, glutathione peroxidase and glucose-6-phosphate dehydrogenase). Serum biochemical markers were significantly higher in paracetamol group as compared to normal control group. Significant increase in oxidative stress parameters and inflammatory mediators was also observed. Treatment with A. marmelos curtailed the toxic effects of paracetamol in a dose dependent fashion. 100 mg/kg dose of A. marmelos was found to be most hepatoprotective. The results of extract-100 group were comparable to silymarin group. Low dose of A. marmelos i.e., 25 mg/kg was combined with piperine to evaluate potentiation of hepatoprotective effects of A. marmelos. Piperine co-administration potentiated the hepatoprotective effects, because the combination group results were comparable to high dose A. marmelos group. A. marmelos exerts hepatoprotective activity through its antioxidant and anti-inflammatory properties which was enhanced by piperine.
Article PDF
Similar content being viewed by others
References
Abirami, A., Nagarani, G., Siddhuraju, P., 2015. Hepatoprotective effect of leaf extracts from Citrus hystrix and C maxima against paracetamol induced liver injury in rats. Food Sci. Hum. Wellness 4, 35–41.
Aebi, H., 1974. Catalase. In: Bergmeyer, H.U. (Ed.), Methods of Enzymatic Analysis. Academic Press, New York, pp. 673–677.
Atal, N., Bedi, K.L., 2010. Bioenhancers: revolutionary concept to market. J. Ayurveda Integr. Med. 1, 96–99.
Athar, M., Iqbal, M., 1998. Ferric nitrilotriacetate promotes N-diethylnitrosamine-induced renal tumorigenesis in the rat: implications for the involvement of oxidative stress. Carcinogenesis 19, 1133–1139.
Baliga, M.S., Bhat, H.P., Josepha, N., Fazala, F., 2011. Phytochemistry and medicinal uses of the bael fruit (Aegle marmelos Correa): a concise review. Food Res. Int. 44, 1768–1775.
Bano, G., Raina, R.K., Zutshi, U., Bedi, K.L., Johri, R.K., Sharma, S.C., 1991. Effect of piperine on bioavailability and pharmacokinetics of propranolol and theophylline in healthy volunteers. Eur. J. Clin. Pharmacol. 41, 615–617.
Benni, J.M., Jayanthi, M.K., Suresha, R.N., 2011. Evaluation of the antiinflammatory activity of Aegle marmelos (Bilwa) root. Indian J. Pharmacol. 43, 393–397.
Bhattacharyya, S., Pence, L., Beger, R., Chaudhuri, S., McCullough, S., Yan, K., Simpson, P., Hennings, L., Hinson, J., James, L., 2013. Acylcarnitine profiles in acetaminophen toxicity in the mouse: comparison to toxicity, metabolism and hepatocyte regeneration. Metabolites 3, 606–622.
Carlberg, I., Mannervik, B., 1975. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 250, 5475–5480.
Chastre, A., Bélanger, M., Beauchesne, E., Nguyen, B.N., Desjardins, P., Butterworth, R.F., 2012. Inflammatory cascades driven by tumor necrosis factor-alpha play a major role in the progression of acute liver failure and its neurological complications. PLoS ONE 7, https://doi.org/10.1371/journal.pone.0049670.
Ellman, G.L., 1959. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82, 70–77.
Ganeshpurkar, A., Saluja, A.K., 2017. The pharmacological potential of rutin. Saudi Pharm. J. 25, 149–164.
Gupta, A.K., Misra, N., 2006. Hepatoprotective activity of aqueous ethanolic extract of Chamomile capitula in paracetamol intoxicated albino rats. Am. J. Pharmacol. Toxicol. 1, 17–20.
Habig, W.H., Pabst, M.J., Jakoby, W.B., 1974. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249, 7130–7139.
Hiwale, A.R., Dhuley, J.N., Naik, S.R., 2002. Effect of co-administration of piperine on pharmacokinetics of beta-lactam antibiotics in rats. Indian J. Exp. Biol. 40, 277–281.
Hussain, L., Ikram, J., Rehman, K., Tariq, M., Ibrahim, M., Akash, M.S.H., 2014. Hepatoprotective effects ofMalvasylvestris L. against paracetamol-induced hep-atotoxicity. Turk. J. Biol. 38, 396–402.
Jaeschke, H., McGill, M.R., Williams, CD., Ramachandran, A., 2011. Current issues with acetaminophen hepatotoxicity - a clinically relevant model to test the efficacy of natural products. Life Sci. 88, 737–745.
Jagetia, G.C., Baliga, M.S., 2004. The evaluation of nitric oxide scavenging activity of certain Indian medicinal plants in vitro: a preliminary study. J. Med. Food 7, 343–348.
Kamalakkannan, N., Prince, P.S.M., 2003a. Effect ofAeglemarmelos Correa (Bael) fruit extract on tissue antioxidants in streptozotocin diabetic rats. Indian J. Exp. Biol. 41, 1285–1288.
Kamalakkannan, N., Prince, P.S.M., 2003b. Hypoglycaemic effect of water extracts of Aegle marmelos fruits in streptozotocin diabetic rats. J. Ethnopharmacol. 87, 207–210.
Kamalakkannan, N., Stanely, M.P.P., 2003. Effect of Aegle marmelos correa fruit extract on tissue antioxidants in streptozotocin diabetic rats. Indian J. Exp. Biol. 41, 285–288.
Khan, T.H., Sultana, S., 2009. Antioxidant and hepatoprotective potential of Aegle marmelos Correa against CCl4-induced oxidative stress and early tumor events. J. Enzyme Inhib. Med. Chem. 24, 320–327.
Koul, I.B., Kapila, A., 1993. Evaluation of the liver protective potential of piperine, an active principle of black and long peppers. Planta Med. 59, 413–417.
Kumari, K.D., Weerakoon, T.C., Handunnetti, S.M., Samarasinghe, K., Suresh, T.S., 2014. Anti-inflammatory activity of dried flower extracts of Aegle marmelos in Wistar rats. J. Ethnopharmacol. 151, 1202–1208.
Lee, E.B., Shin, K.H., Woo, W.S., 1984. Pharmacological study on piperine. Arch. Pharm. Res. 7, 127–132.
Leise, M.D., Poterucha, J.J., Talwalkar, J.A., 2014. Drug-induced liver injury. Mayo Clin. Proc. 89, 95–106.
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J., 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275.
Mahmood, N.D., Mamat, S.S., Kamisan, F.H., Yahya, F., Kamarolzaman, M.F.F., Nasir, N., Mohtarrudin, N., Tohid, S.F., Zakaria, Z.A., 2014. Amelioration of paracetamol-induced hepatotoxicity in rat by the administration of methanol extract of Muntingia calabura L. leaves. Biomed. Res. Int., https://doi.org/10.1155/2014/695678.
Maity, P., Hansda, D., Bandyopadhyay, U, Mishra, D.K., 2009. Biological activities of crude extracts and chemical constituents of Bael, Aegle marmelos (L.) Corr. Indian J. Exp. Biol. 47, 849–861.
Mehmood, M.H., Gilani, A.H., 2010. Pharmacological basis for the medicinal use of black pepper and piperine in gastrointestinal disorders. J. Med. Food 13, 1086–1096.
Mitchell, J.R., Jollow, D.J., Potter, W.Z., Gillette, J.R., Brodie, B.B., 1973. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J. Pharmacol. Exp. Ther. 187, 211–217.
Nelson, S.D., 1990. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin. Liver Dis. 10, 267–278.
Ohkawa, H., Ohishi, N., Yagi, K., 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351–358.
Ozer, J., Ratner, M., Shawc, M., 2008. The current state of serum biomarkers of hepatotoxicity. Toxicology 245, 194–205.
Patil, U.M., Singh, A., Chakraborty, A.K., 2011. Role of piperine as a bioavailability enhancer. Int. J. Recent Adv. Pharm. Res. 1, 16–23.
Radosavljevic, T., Mladenovic, D., Vucevic, D., Vukicevic, R.J., 2010. [The role of oxidative/nitrosative stress in pathogenesis of paracetamol-induced toxic hepatitis]. Med. Pregl. 63, 827–832.
Rajasekaran, C., Kalaivani, T., Ramya, S., Jayakumararaj, R., 2009. Studies on hepatoprotective activity of ethanolic extracts of fruit pulp of Aegle marmelos (L.) Corr. J. Pharm. Res. 2, 1419–1423.
Ravindran, C.A., Murugaiyah, V., Khiang, P.K., Xavior, R., 2013. Hepatoprotective activity of leaf of methanol extract of Laurus nobilis L. against paracetamol induced hepatotoxicity in rats. Asian J. Pharm. Clin. Res. 6, 153–157.
Robak Jm, Gryglewski, R.J., 1988. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 37, 837–841.
Sabina, E.P., Souriyan, A.D.H., Jackline, D., Rasool, M.K., 2010. Piperine, an active ingredient of black pepper attenuates acetaminophen-induced hepatotoxicity in mice. Asian Pac.J. Trop. Med. 3, 971–976.
Sharma, G.N., Dubey, S.K., Sharma, P., Sati, N., 2011. Medicinal values of bael (Aegle marmelos) (L.) Corr.: a review. Int. J. Curr. Pharm. Rev. Res. 1, 12–22.
Singh, H., Sidhu, S., Chopra, K., Khan, M.U., 2016. Hepatoprotective effect of trans-chalcone on experimentally induced hepatic injury in rats: inhibition of hepatic inflammation and fibrosis. Can. J. Physiol. Pharmacol. 94, 879–887.
Tahir, M., Rehman, M.U., Lateef, A., Khan, R., Khan, A.Q., Qamar, W., Ali, F., O’Hamiza, O., Sultana, S., 2013. Diosmin protects against ethanol-induced hepatic injury via alleviation of inflammation and regulation ofTNF-a and NF-kB activation. Alcohol 47, 131–139.
Veerappan, A., Miyazaki, S., Kadarkaraisamy, M., Ranganathan, D., 2007. Acute and subacute toxicity studies of Aegle marmelos Corr., an Indian medicinal plant. Phytomedicine 14, 209–215.
Verma, S., Neil, K., 2009. Diagnosis, management and prevention of drug-induced liver injury. Gut 58, 1555–1564.
Zaheer, N., Tewari, K.K., Krishnan, P.S., 1965. Exposure and solubilization of hepatic mitochondrial shunt dehydrogenases. Arch. Biochem. Biophys. 109, 646–648.
Zu, Y., Fu, Y., Zhao, C., 2006. Simultaneous determination of catechin, rutin, quercetin, kampferol and isorhamnetin in the extracts of sea buckthorn (Hip-pophae rhamnoides L.) leaves by RP-HPLC with DAD. J. Pharm. Biomed. Anal. 41, 714–719.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rathee, D., Kamboj, A., Sachdev, R.K. et al. Hepatoprotective effect of Aegle marmelos augmented with piperine co-administration in paracetamol model. Rev. Bras. Farmacogn. 28, 65–72 (2018). https://doi.org/10.1016/j.bjp.2017.11.003
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.bjp.2017.11.003