Abstract
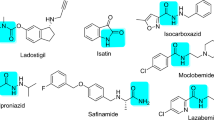
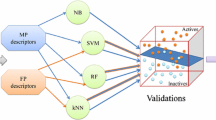
Natural marine products can help increase the quality of life in patients with neurological diseases. A large number of marine products act against Alzheimer’s disease through varying pathways. According to structure- and ligand-based analyses, caulerpin, an alkaloid primarily isolated from the genus Caulerpa, possesses activity against monoamine oxidase B. To predict the activity of caulerpin, we employed Volsurf descriptors and the machine learning Random Forest algorithm in parallel with a structure-based methodology that included molecular docking. Using caulerpin as a lead compound, a database containing 108 analogs was evaluated, and nine were selected as active. The structures selected as active exhibited polar and non-polar substitutions on the caulerpin skeleton, which were relevant for their activity. Dragon consensus drug-like scoring was applied to identify the active analogs that might serve as good drug candidates, and the entire group presented satisfactory performance. These results indicate the possibility of using these analogs as potential leads against Alzheimer’s disease.
Article PDF
Similar content being viewed by others
References
Baunbaek, D., Trinkler, N., Ferandin, Y., Lozach, O., Ploypradith, P., Rucirawat, S., Ishibashi, F., Iwao, M., Meijer, L., 2008. Anticancer alkaloid lamerallins inhibit protein kinase. Mar. Drugs 6, 514–527.
Berthold, M.R., Cebron, N., Dill, F., Gabriel, T.R., Kötter, T., Meinl, T., Ohl, P., Sieb, C., Thield, K., Wiswedel, B., 2007. In: Preisach, C., Burkhrdt, H., Schimidt-Thieme, L., Decker, R. (Eds.), Data analysis, machine learning and applications. Springer, Berlin, pp. 319–326.
Bidon-Chanal, A., Fuertes, A., Alondo, D., Perez, D.I., Martinez, A., Luque, F.J., Medina, M., 2013. Evidence fora new binding mode toGSH-3ß: allosteric regulation by the marine compound palinurin. Eur. J. Med. Chem. 60, 479–489.
Binda, C., Aldeco, M., Geldenhuys, W.J., Tortorici, M., Mattevi, A., Edmondson, D.E., 2012. Molecular insights into human Monoamine Oxidase B inhibition by the glitazone anti-diabetes drugs. Med. Chem. Lett. 3, 39–42.
Binda, C., Milczek, E.M., Bonivento, D., Wang, J., Mattevi, A., Edmondson, D.E., 2011. Lights and shadows on monoamine oxidase inhibition in neuroprotective pharmacological therapies. Curr. Top. Med. Chem. 11, 2788–2796.
Bolea, I., Gella, A., Unzeta, M., 2013. Propargylamine-derived multitarget-directed ligands: fighting Alzheimer’s disease with monoamine oxidase inhibitors. J. Neural Transm. 120, 893–902.
Breiman, L., 2001. Random forests. Mach. Learn. 45, 5–32.
Cavalcante-Silva, L.H.A., Correia, A.C.C., Barbosa-Filho, J.M., Silva, B.A., Santos, B.V.O., Lira, D.P., Miranda, G.E.C., Cavalcante, F.A., Moreira, M.S.A., 2013. Spasmolytic effect of caulerpin involves blockade of Ca influx on guinea pig ileum. Mar. Drugs 11, 1553–1564.
Chen, G., Zheng, S., Luo, X., Shen, J., Zhu, W., Liu, H., Gui, C., Zhang, J., Zheng, M., Puah, C.M., Chen, K., Jiang, H., 2005. Focused combinatorial library design based on structural diversity, druglikeness and binding affinity score. J. Comb. Chem. 7, 398–406.
Choi, B.W., Ryu, G., Park, S.H., Kim, E.S., Shin, J., Roh, S.S., Shin, H.C., Lee, B.H., 2007. Anticholinesterase activity of plastoquinones from Sargassum sagamianum: lead compounds for Alzheimer’s diseases therapy. Phytother. Res. 21, 423–426.
Choi, D.Y., Choi, H., 2015. Natural products from marine organisms with neuroprotective activity in the experimental models of Alzheimer’s disease, Parkinson’s disease and ischemic brain stroke: their molecular targets and action mechanisms. Arch. Pharm. Res. 38, 139–170.
Cruciani, G., Crivori, P., Carrupt, P.A., Testa, B., 2000. Molecular fields in quantitative structure-permeation relationships: the Volsurf approach. J. Mol. Struc. Theochem. 503, 17–30.
Custódio, L., Justo, T., Silvestre, L., Barradas, A., Duarte, C.V., Pereira, H., Barreira, L., Rauter, A.P., Alberío, F., Varela, J., 2012. Microalgae of different phyla display antioxidant, metal chelating and acetylcholinesterase inhibitors activities. Food Chem. 131, 134–140.
Debdab, M., Renault, S., Lozach, O., Meijer, L., Paquin, L., Carreaux, F., Bazureau, J.P., 2010. Synthesis and preliminary biological evaluation of new derivatives ofthe marine alkaloid leucettamine B as kinase inhibitors. Eur. J. Med. Chem. 45, 805–810.
Fernandez, H.H., Chen, J.J., 2007. Monoamine oxidase-B inhibition in the treatment of Parkinson’s disease. Pharmacotherapy 27, 174–185.
Gerwick, W.H., Moore, B.S., 2012. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 19, 85–98.
Gul, W., Hamann, M.T., 2005. Indole alkaloid marine natural products: an established source of cancer drugs leads with considerable promise forthe control of parasitic, neurological and other diseases. Life Sci. 78, 442–453.
Hall, M., Fran K. E., Holmes, G., Pfahringer, B., Reutemann, P., Witten, I.H., 2009. The WEKAdata mining software: an update. SIGKDD Exploration 11.
Hamann, M., Alonso, D., Martin-Aparicio, E., Fuertes, A., Pérez-Puerto, M.J., Castro, A., Morales, S., Navarro, M.L., Del Monte-Millán, M., Medina, M., Pennaka, H., Balaiah, A., Peng, J., Cook, J., Wahyuono, S., Martínez, A., 2007. Glycogen synthase kinase (GSK-3ß) inhibitory activity and structure activity relationship (SAR) studies of themanzamine alkaloids. Potencial for Alzheimer’s disease. J. Nat. Prod. 70, 1397–1405.
Hanley, J.A., McNeil, B.J., 1982. The meaning and use ofthe area under a receiver operation characteristic (ROC) curve. Radiology 143, 29–36.
Imre, G., Veressc, G., Volfordd, A., Farkas, Ö., 2003. Molecules from the Minkowski space: an approach to building 3D molecular structure. J. Mol. Struc. Theochem. 666, 51–59.
Jin, D.Q., Lim, C.S., Sung, J.Y., Choi, H.G., Ha, I., Han, J.S., 2006. Ulva conglabata, a marine algae, has neuroprotective and antiinflammatory effects in murine hippocampal and microglial cells. Neurosci. Lett. 402, 154–158.
LaFerla, F.M., Oddo, S., 2005. Alzheimer’s disease: abeta, tau and synaptic dysfunction. Trends Mol. Med. 11, 170–176.
Langjae, R., Bussarawit, S., Yuenyongsawad, S., Ingkaninan, K., Plubrukarn, 2007. Acetylcholinesterase-inhibiting steroidal alkaloid from the sponge Corticium sp. Steroids 72, 682–685.
Lill, M.A., Danielson, M.L., 2011. Computer-aided drug design platform using PyMOL. J. Comput. Aided Mol. Des. 25, 13–19.
Lipinski, C.A., Lombardo, F., Dominy, B.W., Feeney, P.J., 2001. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliver. Rev. 46, 3–26.
Liu, C.-C., Kanekiyo, T., Xu, H., Bu, G., 2013. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 9, 106–118.
Mayer, A.M.S., Rodrígiez, A.D., Berlink, R.G.S., Fusetani, N., 2011. Marine pharmacology in 2007–8: marine compounds with antibacterial, anticoagulant, antifungal, antiinflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanism of action. Comp. Biochem. Physiol. 153, 191–222.
Meijer, L., Thunnissen, A.M.W.H., White, A.W., Garnier, M., Nokolic, M., Tsai, L.H., Walter, J., Cleverley, K.E., Salinas, P.C., Wu, Y.Z., Bienart, J., Mandelkow, E-M., Kim, S.H., Petit, G.R., 2000. Inhibition of cyclin-dependet kinase, GSK-3ß and CSK1 by hymenialdisine, a marine sponge constituent. Chem. Biol. 7, 51–63.
Meijer, L., Skaltsounis, A.L., Magiatis, P., Polychronopoulus, P., Knockaert, M., Leost, M., Ryan, X.P., Vonica, C.A., Brivanlou, A., Dajani, R., Crovace, C., Tarricone, C., Musacchio, A., Roe, S.M., Pearl, L., Greengard, P., 2003. GSK-3 selective inhibitors derived from tyrian purple indirubins. Chem. Biol. 10, 1255–1266.
Motohashi, K., Toda, T., Sue, M., Furihata, K., Shizuri, Y., Matsuo, Y., Kasai, H., Shinya, K., Takagi, M., Izimikawa, M., Horikawa, Y., Seto, H., 2010. Isolation and structure elucidation of tumescenamides A and B, two peptides produced by Streptomyces tumescens YM23-260. J. Antibiot. 63, 549–552.
Oprea, T.I., Sherbukhin, V., Svensson, P., Kuhler, T.C., 2000. Chemical information management in drug discovery: optimizing the computational and combinatorial chemistry interfaces. J. Mol. Graph. Model. 18, 512–524.
Patil, P.O., Bari, S.B., Firke, S.D., Deshmukh, P.K., Donda, S.T., Patil, D.A., 2013. A comprehensive review on synthesis and designing aspects of coumarin derivatives as monoamine oxidase inhibitors for depression and Alzheimer’s disease. Bioorg. Med. Chem. 21, 2434–2450.
Radwan, M.A., El-Sherbiny, M., 2007. Synthesis and antitumor activity of indolylpyrimidines: marine natural product meridianin D analogues. Bioorg. Med. Chem. 15, 1206–1211.
Riediger, N.D., Othman, R.A., Suh, M., Moghadasian, M.H., 2009. A systemic review of the roles of n-3 fattyacid in health and diseases. J. Am. Diet. Assoc. 109, 669–679.
Rishton, G.M., 2003. Nonleadlikeness and leadlikeness in biochemical screening. Drug Discovery Today 8, 86–96.
Schumacher, M., Kelkel, M., Dicato, M., Diederich, M., 2011. Gold from the sea: marine compounds inhibitors ofthe hallmarks of cancer. Biotechnol. Adv. 29, 531–547.
Scotti, L., Fernandez, M.B., Muramatsu, E., Pasqualoto, K.F.M., Emereciano, V.D., Tavares, L.C., Silva, M.S., Scotti, M.T., 2011. Self-organizing maps and Volsurf approach to predict aldose reductase inhibition by flavonoid compounds. Rev. Bras. Farmacog. 21, 170–180.
Souza, E.T., Queiroz, A.C., Miranda, G.E.C., Lorenzo, V.P., Silva, E.F., Freire-Dias, T.L.M., Cupertino-Silva, Y.K., Melo, G.M.A., Santos, B.V.O., Chaves, M.C.O., Alexandre-Moreira, M.S., 2009a. Antinociceptive activities of crude methanolic extract and phases, n-butanolic, chloroformic and ethyl acetate from Caulerpa racemosa (Caulerpaceae). Rev. Bras. Farmacog. 19, 115–120.
Souza, E.T., Pereira, D.L., Queiroz, A.C., Miranda, G.E.C., Lorenzo, V.P., Silva, D.J.C., Bezerra, A.A., Campessato, E.A.M., Araújo-Júnior, J.X., Barbosa-Filho, J.M., Athayde-Filho, P.F., Santos, B.V.O., Chaves, M.C.O., Alexandre-Moreira, M.S., 2009b. The antinociceptive and antiinflammatory activities of Caulerpin, a bisindole alkaloid isolated from seaweeds ofthe genus Caulerpa. Mar. Drugs 7, 689–704.
Talete srl, Dragon (Software for Molecular Descriptor Calculation) Version 6.0. (2014). http://www.talete.mi.it/.
Thomas, N.V., Kim, S.K., 2011. Potential pharmacological applications of polyphenolic derivatives from marine brown algae. Environ. Toxicol. Pharmacol. 32, 325–335.
Thomsen, R., Christensen, M.H., 2006. Moldock: a new technique for high-accuracy molecular docking. J. Med. Chem. 49, 3315–3321.
Tian, L.W., Feng, Y., Shimizu, Y., Pfeifer, T.A., Wellington, C., Hooper, J.N.A., Quinn, R.J., 2014. ApoE secretion modulating bromotyrosine derivate from the Australian marine sponge Callyspongia sp. Bioorg. Med. Chem. Lett. 24, 3537–3540.
Turk, T., Avgustin, J.A., Batista, U., Strugar, G., Kosmina, R., Civovic, S., Janussen, D., Kauferstein, S., Mebs, D., Sepcic, K., 2013. Biological activities of ethanolic extracts from deep sea Antarctic marine sponges. Mar. Drugs 11, 1126–1139.
Veber, D.F., Johnson, S.R., Cheng, H.Y., Smith, B.R., Ward, K.W., Kopple, K.D., 2002. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 12, 2615–2623.
Walters, W.P., Murcko, M.A., 2002. Prediction of‘drug-likeness’. Adv. Drug Deliv. Rev. 54, 255–271.
Wyss-Coray, T., Rogers, J., 2012. Inflammation in Alzheimer disease - abrief review ofthe basic science and clinical literature. Cold Spring Harb. Perspect. Med. 2, a006346.
Yang, C.G., Liu, G., Jiang, B., 2002. Preparing functional bis (indole) pyrazine by stepwise cross-coupling reactions: an efficient method to construct the skeleton of dragmacidin D.J. Org. Chem. 67, 9392–9396.
Yoon, N.Y., Lee, S.H., Li, Y., Kim, S.K., 2009. Phlorotannins from Ishige okamurae and theiracetylandbutylcholinesterase inhibitors effects. J. Func. Foods 1, 331–335.
Zheng, S., Luo, X., Chen, G., Zhu, W., Shen, J., Chen, K., Jiang, H., 2005. A new rapid and effective chemistry space filter in recognizing a druglike database. J. Chem. Inf. Model. 45, 856–862.
Zhu, W., Xie, W., Pan, T., Jankovic, J., Li, J., Youdim, M.B., Le, W., 2008. Comparison of neuroprotective and neurorestorative capabilities of rasagiline and selegiline against lactacystin-induced nigrostriatal dopaminergic degeneration. J. Neurochem. 105, 1970–1978.
Acknowledgment
The authors would like to thank the Brazilian National Counsel of Technological and Scientific Development (CNPq) for financial support.
Author information
Authors and Affiliations
Contributions
VPL produced the analogs and performed the docking study. MTS and LS created the ligand based model. All of the authors have read the final manuscript and have agreed to its submission for appraisal.
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lorenzo, V.P., Filho, J.M.B., Scotti, L. et al. Combined structure- and ligand-based virtual screening to evaluate caulerpin analogs with potential inhibitory activity against monoamine oxidase B. Rev. Bras. Farmacogn. 25, 690–697 (2015). https://doi.org/10.1016/j.bjp.2015.08.005
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.bjp.2015.08.005