Abstract
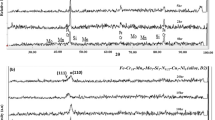
The passivation behaviors of super martensitic stainless steels (SMSS) were studied by polarization curves at passive potential of −0.1 V and in various NaCl solutions, electrochemical impedance spectroscopy (EIS) and X-ray photoelectron spectroscopy (XPS) analysis. Electrochemical test results showed that, in alkaline solutions, passivation region width was wider, passivation current was smaller, and polarization resistance was greater; thus, the passive film of SMSS in alkaline solutions had better passivation behaviors than that in acidic solutions. The polarization curve and EIS of samples SMSS1 and SMSS2 were also used to study which sample had better passivation behaviors. All results demonstrated that passive film structure of SMSS1 sample was more stable, and capacity of passive film was enhanced. The impact of alloying elements on the passive film (SMSS) passivation capability was also discussed by XPS depth profiling, and XPS depth profiling showed that the composition of the passive film was mainly composed of Fe-oxide and Cr-oxide. So the passive film structures were mixed layers of Fe-oxide and Cr-oxide. Fe oxidation product and Cr oxidation product would help to improve the protective property of passive film, which could promote the formation of a passive film structure more stably and densely.
Similar content being viewed by others
References
A. Kocijan, C. Donik, M. Jenko, Corros. Sci. 49 (2007) 2083–2098.
M. F. Montemor, M. G. S. Ferreira, N. E. Hakiki, Corros. Sci. 42 (2000) 1635–1650.
S. Mischler, A. Vogel, H. J. Mathieu, D. Landolt, Corros. Sci. 32 (1991) 925–944.
M. Sánchez, H. Mahmoud, J. Solid State Electr. 16 (2012) 1193–1202.
D. N. Zou, R. Liu, J. Li, W. Zhang, D. Wang, Y. Han, J. Iron Steel Res. Int. 21 (2014) No. 6, 630–636.
X. C. Han, J. Li, K. Y. Zhao, W. Zhang, J. Su, J. Iron Steel Res. Int. 20 (2013) No. 5, 74–79.
C. O. A. Olsson, D. Landolt, Electrochim. Acta 48 (2003) 1093–1104.
S. Y. Kim, H. Kim, H. S. Kwan, Mater. Corros. 57 (2006) 835–842.
S. Haupt, H. H. Strehblow, Corros. Sci. 37 (1995) 43–54.
H. W. Hoppe, S. Haupt, H. H. Strehblow, Surf. Interface Anal. 21 (1994) 514–525.
Z. Petrovic, N. Lajçi, M. Metikoš-Hukovic, R. Babic, J. Solid State Electr. 15 (2010) 1201–1207.
J. Ding, L. Zhang, M. Lu, J. Wang, Z. Wen, W. Hao, Appl. Surf. Sci. 289 (2014) 33–41.
A. Davoodi, M. Pakshir, M. Babaiee, G. R. Ebrahimi, Corros. Sci. 53 (2011) 399–408.
E. James, Concrete Eng. Int. 6 (2002) 64–67.
D. V. Val, M. G. Stewart, Struct. Saf. 25 (2003) 343–362.
K. M. Kim, K. Y. Kim, J. Power Sources 173 (2007) 917–924.
W. Jiang, K. Y. Zhao, D. Ye, J. Li, Z. D. Li, J. Su, J. Iron Steel Res. Int. 20 (2013) No. 5, 61–65.
D. Ye, J. Li, W. Jiang, J. Su, K. Zhao, Mater. Des. 41 (2012) 16–22.
Y. R. Liu, D. Ye, Q. L. Yong, J. Su, K. Y. Zhao, W. Jiang, J. Iron Steel Res. Int. 18 (2011) No. 11, 60–66.
Y. F. Chen, J. L. Luo, Electrochim. Acta 44 (1999) 2947–2957.
A. Saatchi, M. A. Golozar, K. Raeissi, J. Appl. Electrochem. 40 (2010) 457–461.
R. S. Lillard, G. S. Kanner, L. L. Daemen, Electrochim. Acta 47 (2002) 2473–2482.
C. Boissy, C. A. Dumont, B. Normand, Electrochem. Commun. 26 (2013) 10–12.
A. A. Hermas, M. S. Morad, Corros. Sci. 50 (2008) 2710–2717.
B. Guitián, X. R. Nóvoa, B. Puga, Electrochim. Acta. 56 (2011) 7772–7779.
H. Luo, C. F. Dong, K. Xiao, X. G. Li, Appl. Surf. Sci. 258 (2011) 631–639.
V. Guiñón-Pina, A. Igual-Muñoz, J. García-Antón, Corros. Sci. 53 (2011) 575–581.
A. Kocijan, D. K. Merl, M. Jenko, Corros. Sci. 53 (2011) 776–783.
R. A. Antunes, M. C. L. Oliveira, G. Ett, V. Ett, Int. J. Energ. Res. 36 (2011) 12474–12485.
A. Popova, E. Sokolova, S. Raicheva, M. Christov, Corros. Sci. 45 (2003) 33–58.
A. K. Iversen, Corros. Sci. 48 (2006) 1036–1058.
Z. J. Zheng, Y. Gao, Y. Gui, M. Zhu, J. Solid State Electr. 18 (2014) 2201–2210.
L. A. S. Ries, M. Da Cunha Belo, M. G. S. Ferreira, L. L. Muller, Corros. Sci. 50 (2008) 676–686.
C. Hitz, A. Lasia, J. Electroanal. Chem. 500 (2001) 213–222.
C. F. Chen, R. J. Jiang, J. S. Qian, S. Q. Zheng, Acta Phys. Chim. Sin. 25 (2009) 1213–1218.
A. Gebert, K. Buchholz, A. Leonhard, K. Mummert, J. Eckert, L. Schultz, Mater. Sci. Eng. A 267 (1999) 294–300.
M. J. Carmezim, A. M. Simões, M. F. Montemor, M. Da Cunha Belo, Corros. Sci. 47 (2005) 581–591.
S. Nagarajan, N. Rajendran, Corros. Sci. 51 (2009) 217–224.
Y. H. Lin, R. G. Du, R. G. Hu, C. J. Lin, Acta Phys. Chim. Sin. 21 (2005) 740–745.
A. Irhzo, Y. Segui, N. Bui, F. Dabosi, Corros. Sci. 26 (1986) 769–780.
J. S. Kim, P. J. Xiang, K. Y. Kim, Corrosion 61 (2005) 174–183.
K. M. Kim, J. H. Kim, K. Y. Kim, ECS Trans. 25 (2009) 1823–1832.
K. H. Lo, C. H. Shek, J. K. L. Lai, Mater. Sci. Eng. R 65 (2009) 39–104.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation Item: Item Sponsored by Education Department Scientific Research Fund Project from Yunnan Province of China (2012Y544)
Rights and permissions
About this article
Cite this article
Kang, J., Li, J., Zhao, Ky. et al. Passivation Behaviors of Super Martensitic Stainless Steel in Weak Acidic and Weak Alkaline NaCl Solutions. J. Iron Steel Res. Int. 22, 1156–1163 (2015). https://doi.org/10.1016/S1006-706X(15)30127-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1016/S1006-706X(15)30127-8