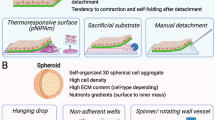
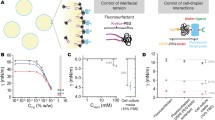
Abstract
Cells tend to form hierarchy structures in native tissues. Formation of cell aggregates in vitro such as cancer spheroids and embryonic bodies provides a unique means to study the mechanical properties and biological behaviors/functions of their counterparts in vivo. In this paper, we review state-of-the-art experimental approaches to assess the mechanical properties and mechanically-induced responses of cell aggregates in vitro. These approaches are classified into five categories according to loading modality, including micropipette aspiration, centrifugation, compression loading, substrate distention, and fluid shear loading. We discussed the advantages and disadvantages of each approach, and the potential biomedical applications. Understanding of the mechanical behavior of cell aggregates provides insights to physical interactions between cells and integrity of biological functions, which may enable mechanical intervention for diseases such as atheromatosis and cancer.
Similar content being viewed by others
References
Guevorkian, K., Colbert, M.J., Durth, M., Dufour, S. and Brochard-Wyart, F., Aspiration of biological viscoelastic drops. Physical Review Letters, 2010, 104(21): 1–4.
Hughes, L.C., Archer, C.W. and Gwynn, I.A., The ultrastructure of mouse articular cartilage: collagen orientation and implications for tissue functionality. European Cells and Materials, 2005, 9: 68–84.
Ulitsky, I., Sive, H., Jan, C.H., Shkumatava, A. and Bartel, D.P., Conserved function of lincRNAs in vertebrate embryonic development despite rapid sequence evolution. Elsevier Science, 2011, 147(7): 1537–1550.
Smid, M., Wang, Y., Zhang, Y.X., Klijn, J.G.M., Siewerts, A.M., Atkins, D., Martens, J.W.M. and Foekens, J.A., Genes associated with breast cancer metastatic to bone. Journal of Clinical Oncology, 2006, 24(15): 2261–2267.
Powers, M.J., Janigian, D.M., Wack, K.E., Baker, C.S., Stolz, D.B. and Griffitch, L.G., Functional behavior of primary rat liver cells in a three-dimensional perfused microarray bioreactor. Tissue Engineering, 2004, 8(3): 499–513.
Krieg, M., Arboleda-Estudillo, Y. and Puech, P.H., Tensile forces govern germ-layer organization in zebrafish. Nature Cell Biology, 2008, 10(4): 429–436.
Xu, F., Sridharan, B. and Wang, S.Q., Cell printing for controlled-size embryoid body formation. Biomicrofluidics, 2011: p. doi: 10.1063/1.3580752.
Meads, M.B., Gatenby, R.A. and Dalton, D.S., Environment-mediated drug resistance: a major contributor to minimal residual disease. Nature Reviews Cancer, 2009, 9(9): 665–674.
Chen, L., Park, S.M., Tumanov, A.V., Hau, A., Sawada, K., Feig, C., Turner, J.R., Fu, Y.X., Romero, I.L., Lengyel, E. and Peter, M.E., CD95 promotes tumour growth. Nature, 2010, 465: 492–426.
Liotta, L.A. and Kohn, E.C., The microenvironment of the tumour-host interface. Nature, 2001, 411(6835): 375–379.
Engler, A.J., Sen, S., Sweeney, H.L. and Discher, D.E., Matrix elasticity directs stem cell lineage specification. Cell, 2006, 126(4): 677–689.
Battista, S., Guarnieri, D., Borselli, C., Zeppetelli, S., Borzacchiello, A., Mayol, L., Gerbasio, D., Keene, D.R., Ambrosio, L. and Netti, P.A., The effect of matrix composition of 3D constructs on embryonic stem cell differentiation. Biomaterials, 2005, 26(31): 6194–6207.
Butcher, D.T., Alliston, T. and Weaver, V.M., A tense situation: forcing tumour progression. Nature Reviews Cancer, 2009, 9(2): 108–122.
Huang, S. and Ingber, D.E., Cell tension, matrix mechanics, and cancer development. Cancer Cell, 2005, 8(3): 175–176.
Watanabe, S., Sato, S., Ohtsuka, K. and Takenaka, S., Electrochemical DNA analysis with a supramolecular assembly of naphthalene diimide, ferrocene, and beta-cyclodextrin. Analytical Chemistry, 2011, 83(19):7290–7296.
Xu, F., Wu, C.M., Rengarajan, V., Finley, T.D., Keles, H.O., Sung, Y., Li, B., Gurkan, U.A. and Demirci, U., Three-dimensional magnetic assembly of microscale hydrogels. Advanced Materials, 2011, 23(37): 4254–4260.
Xu, F., Finley, T.D., Turkaydin, M., Sung, Y., Gurkan, U.A., Yavuz, A.S., Guldiken, R. and Demirci, U., The assembly of cell-encapsulating microscale hydrogels using acoustic waves. Biomaterials, 2011, 32(31): 7847–7855.
Hochmuth, R.M., Micropipette aspiration of living cells. Journal of Biomechanics, 2000, 33(1): 15–22.
Pandey, R., Gupta, R.K., Shahid, M., Maiti, B., Misra, A. and Pandey, D.S., Synthesis and characterization of electroactive ferrocene derivatives: ferrocenylimidazoquinazoline as a multichannel chemosensor selectively for Hg2+ and Pb2+ ions in an aqueous environment. Inorganic Chemistry, 2012, 51(1):298–311.
Zhou, E.H., Xu, F., Quek, S.T. and Lim, C.T., A power-law rheology based finite element model for single cell deformation. Biomechanics Modeling Mechanobiology, DOI: 10.1007/s10237-012-0374-y, 2012.
Guevorkiana, K., D.Gonzalez-Rodriguez, D., Carlierb, C., Dufour, S. and Brochard-Wyart, F., Mechanosensitive shivering of model tissues under controlled aspiration. PNAS, 2011, 108(33): 13387–13392.
Guevorkian, K.C., Durth, M.J. and Durth, M.L., Aspiration of biological viscoelastic drops. Physical Review Letters, 2010, 104: 1–4.
Daniel, S.J. and N.Chao, L., Noncontact measurement of the local mechanical properties of living cells using pressure applied via a pipette. Biophysical Journal, 2008, 95(6): 3017–3027.
Forgacs, G.K. and Kosztin, I., Cellular aggregates under pressure. American Physical Society, 2010, 3(43): 3017–3027.
Marmottanta, P.M. and Audrenb, B., The role of fluctuations and stress on the effective viscosity of cell aggregates. PNAS, 2009, 106(41): 17271–17275.
Kalantarian, A., Ninomiya, H., Saad, S.M., David, R., Winklbauer, R. and Neumann, A.W., Axisymmetric drop shape analysis for estimating the surface tension of cell aggregates by centrifugation. Biophysical Journal, 2009, 96(4): 1606–1616.
Evseenko, D.D. and Zhu, G., Identification of the Critical Extracellular Matrix Proteins that Promote Human Embryonic Stem Cell Assembly. Stem Cells and Development, 2009, 18(6): 919–928.
Liu, C.Z. and Wang, Y., Effects of cyclic hydraulic pressure on osteocytes. International Bone and Mineral Society, 2010, 46(5): 1449–1456.
Elder, B.D., Hydrostatic pressure in articular cartilage tissue engineering: from chondrocytes to tissue regeneration. Tissue Engineering, 2009, 15(1): 43–53.
Liu, L.L. and Liao, D., A microfluidic device for continuous cancer cell culture and passage with hydrodynamic forces. Lab on a Chip, 2010, 10(14): 1807–1813.
Shahin, K.D., Tissue engineering of cartilage using a mechanobioreactor exerting simultaneous mechanical shear and compression to simulate the rolling action of articular joints. Biotechnology and Bioengineering, 2012, 109(4): 1060–1073.
Thorpe, S.D.B. and Vinardell, T., The response of bone marrow-derived mesenchymal stem cells to dynamic compression following TGF-β3 induced chondrogenic differentiation. Annals of Biomedical Engineering, 2010, 38(9): 2896–2909.
Dunkers, J.C. and Pakstis, L., Solutions for determining equibiaxial substrate strain for dynamic cell culture. Journal of Biomechanics, 2010, 43(13): 2613–2617.
Yu, H.Y.R. and Sandham, A., Mechanical tensile stress effects on the expression of bone sialoprotein in bovine cementoblasts. The Angle Orthodontist, 2009, 79(2): 346–352.
Durst, C.A. and Mansfield, E.G., Flexural characterization of cell encapsulated PEGDA hydrogels with applications for tissue engineered heart valves. Acta Biomaterialia, 2011, 7(6): 346–352.
Poapongsakorn, P., Time-dependent deformation of closed-cell PVC foam. Journal of Cellular Plastics, 2011, 47(4): 323–336.
Ferdousa, Z.J. and Nerem, R.M., Differences in valvular and vascularcell responses to strain in osteogenic media. Biomaterials, 2011, 32(11): 2885–2893.
Connolly, S.C.S. and Fairbank, N.J., Chronic oscillatory strain induces MLCK associated rapid recovery from acute stretch in airway smooth muscle cells. Journal of Applied Physiology, 2011, 111(4): 955–963.
Bartalena, G.G. and Sharma, R.I., A novel method for assessing adherent single-cell stiffness in tension: design and testing of a substrate-based live cell functional imaging device. Biomedical Microdevices, 2011, 13(2): 291–301.
Muravyov, A.V.T. and Maimistova, A.A., Extra- and intracellular signaling pathways under red blood cell aggregation and deformability changes. Clinical Hemorheology and Microcirculation, 2009, 43(3): 223–232.
Huang, L.M. and Helmke, B.P., A stretching device for high-resolution live-cell imaging. Annals of Biomedical Engineering, 2010, 38(5): 1728–1740.
Cheng, T.P. and Dunkers, J., Solutions for determining equibiaxial substrate strain for dynamic cell culture. Journal of Biomechanics, 2010, 43(13): 2613–2615.
Andrew, A. and Pitsillides, S.C., Using cell and organ culture models to analyze responses of bone cells to mechanical stimulation. Bone Research Protocols, 2012, 816(7): 593–619.
Billiar, K.L., The mechanical environment of cells in collagen gel models global and local effects in three-dimensional biological hydrogels. Tissue Engineering and Biomaterials, 2011, 4: 593–619.
Sander, E.A. and Swickrath, M.J., Out of many, one: modeling schemes for biopolymer and biofibril networks. Chemistry and Physics Science, 2010, 9: 557–602.
Vandenburghc, H. and Yunga, Y.C., Cellular strain assessment tool (CSAT): precision-controlled cyclic uniaxial tensile loading. Journal of Biomechanics, 2009, 42(2): 178–182.
Shin, H.S. and Sim, S.J., Shear stress effect on transfection of neurons cultured in microfluidic devices. Journal of Nanoscience and Nanotechnology, 2009, 9: 1–6.
Schwartz, G. and Uhricha, C., Efficient and long-term stable organic vacuum deposited tandem solar cells. Proceedings of SPIE Digital Library, 2009, 7416: 1–11.
Yamamoto, K., Shear stress mechanotransduction via endogenous ATP release in vascular endothelial cells. IFMBE Proceedings, 2012, 37(1): 777–780.
Yamamoto, J.A., Shear-stress-sensing and response mechanisms in vascular endothelial cells. Interface Oral Health Science, 2010, 1: 3–9.
Obi, S. and Shimizu, N., Fluid shear stress induces arterial differentiation of endothelial progenitor cells. Journal of Applied Physiology, 2009, 106(1): 203–211.
Brown, A. and Brian, J., Modeling of shear stress experienced by endothelial cells cultured on microstructured polymer substrates in a parallel plate flow chamber. Biotechnology and Bioengineering, 2011, 108(5): 1148–1158.
Condorelli, L. and Arrigoni, C., Effect of fluid shear stress on tubular kidney epithelial cell structure. IFMBE Proceedings, 2010, 25(10): 50–52.
Huesa, C. and Richard, M., Parallel-plate fluid flow systems for bone cell stimulation. Journal of Biomechanics, 2010, 43(6): 1182–1189.
Wang, X.Q. and Fujiwara, K., Thioredoxin interacting protein promotes endothelial cell inflammation in response to disturbed flow by increasing leukocyte adhesion and repressing kruppel-like factor 2. Circulation Research, 2012, 110(4): 560–568.
Dreyer, L. and Autschbach, R., An advanced cone-and-plate reactor for the in vitro-application of shear stress on adherent cells. Clinical Hemorheology and Microcirculation, 2011, 49(1): 391–397.
Obi, S. and Masumura, T., Shear stress induces arterial differentiation of bone marrow-derived endothelial progenitor cells. Micro-NanoMechatronics and Human Science, 2009, 9: 650–655.
Chiu, J.J. and Chien, S., Vascular endothelial responses to altered shear stress: Pathologic implications for atherosclerosis. Annals of Medicine, 2009, 41(1): 19–28.
Geitmann, A., How to shape a cylinder: pollen tube as a model system for the generation of complex cellular geometry. Sexual Plant Reproduction, 2010, 23(1): 63–71.
Tilton, N.M. and Serre, E., Pressure-driven radial flow in a Taylor-Couette cell. Journal of Fluid Mechanics, 2010, 660: 527–537.
Conway, D.E. and Eskin, S.G., Endothelial cell responses to atheroprone flow are driven by two separate flow components: low time-average shear stress and fluid flow reversal. Heart and Circulatory Physiology, 2010, 298(2): 367–374.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Project supported by the Major International (Regional) Joint Research Program of China (11120101002); the National Natural Science Foundation of China (Nos. 10825210 and 81000453); the National 111 Project of China (B06024) and the Natural Science Foundation of Shaanxi Province, China (No. 2012JQ1006). F.Xu was also partially supported by the China Young 1000-Talent Program and Shaanxi 100-Talent Program.
Rights and permissions
About this article
Cite this article
Zhang, W., Wang, S., Lin, M. et al. Advances in Experimental Approaches for Investigating Cell Aggregate Mechanics. Acta Mech. Solida Sin. 25, 473–482 (2012). https://doi.org/10.1016/S0894-9166(12)60042-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1016/S0894-9166(12)60042-1