Abstract
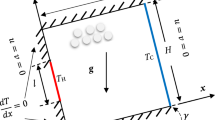
In this work, we solved the differential equations of mass and energy balance describing the n-hexane adsorption and desorption process in 5A zeolite under non-isothermal and non-adiabatic conditions. The solution provided a theoretical model that may be used to determine the significance of the mass and thermal transfer effects applied to transient adsorption. We validated numerical results with experimental data from the literature, finding the n-hexane adsorption to be exothermic. Because the diffusion in the system is fast, the breakthrough curves showed the typical form of thermal limitation. We found desorption depends on the desorbent fluid and feed rate. We evaluated the stability of the numerical method using the eigenvalues of the matrix system. Hence, the computational code developed may be used to simulate real operating conditions for the adsorption/desorption process of gases with porous adsorbents once the process parameters are known.










Similar content being viewed by others
Abbreviations
- a p :
-
Specific area of the pellet, ap = 3/Rp (m2 m−3)
- a c :
-
Specific area of the column, ac = 2/Rc (m2 m−3)
- C f :
-
Concentration at time zero (feed) (kg m−1)
- C pg :
-
Heat capacity of gas (kg m2 s−2 mol−1 K−1)
- C ps :
-
Heat capacity of solid (m2 s−2 K−1)
- D :
-
Source term added the Dirichlet boundary conditions (K)
- D L :
-
Axial dispersion (m2 s−1)
- F :
-
Total molar flux (mol m−2 s−1)
- h p :
-
Film heat-transfer coefficient (kg s−3 K−1)
- h w :
-
Wall heat-transfer coefficient at the wall (kg s−3 K−1)
- J :
-
Total flow rate (kg s−1)
- K L :
-
Effective axial bed thermal conductivity (kg m s−3 K−1)
- K ads :
-
Adsorption equilibrium constant (atm−1)
- k 0 :
-
Parameter of the isotherm of Nitta et al. (1984) (atm−1)
- K gl :
-
Global mass-transfer coefficient (m s−1)
- θ a :
-
Parameter of the isotherm of Nitta et al. (1984) (dimensionless)
- L:
-
Column length (m)
- N :
-
Parameter of the isotherm of Nitta et al. (1984) (dimensionless)
- P 0 :
-
System pressure (atm)
- Q a :
-
Concentration of the chemical species “a” adsorbed in the solid phase (kg kg−1)
- \(\overline{{q_{a} }}\) :
-
Average adsorbent phase concentration (kg kg−1)
- \({q}_{max}\) :
-
Maximum concentration absorbable (kg kg−1)
- R :
-
Ideal gas constant (kcal mol−1 K−1)
- R c :
-
Radius of the column (m)
- R p :
-
Radius of the pellet (m)
- S :
-
Source term (K)
- T :
-
Temperature in the gas phase (K)
- T s :
-
Temperature in the solid phase (K)
- T w :
-
Wall temperature (K)
- t :
-
Time (s)
- u f :
-
Feed velocity (m s−1)
- u :
-
Apparent velocity (m s−1)
- W :
-
Discrete sink term (kg m−1)
- y a :
-
Mole fraction of sorbate in gas phase (mol mol−1)
- \(\bar{y}_{a}\) :
-
Average mole fraction of sorbate in gas phase (mol mol−1)
- y af :
-
Mole fraction of sorbate in gas phase at feed conditions (mol mol−1)
- z:
-
Distance variable in the longitudinal direction of the column (m)
- ɛ b :
-
Bed porosity (dimensionless)
- ΔHads :
-
Isosteric heat of adsorption (kcal mol−1)
- ρ a :
-
Apparent density (kg m−3)
- Δt :
-
Time interval of integration (s)
- Δz :
-
Length of an elementary volume (m)
- t:
-
Time discretization
- z:
-
Axial coordinate in bed (m)
References
Ahmed M, Hussein M (2015) Experimental and theoretical analysis of iso-butane recovery from linear paraffinic hydrocarbons by adsorption on 5A zeolite. J Nat Gas Sci Eng 27(2):763–768
Barrer RM (1981) Sorption in porous crystals: equilibria and their interpretation. J Chem Technol Biotechnol 31:71
Çengel YA (2011) Heat and mass transfer: a practical approach. Mc-Graw Hill Education, Columbus, GA, USA
Chapra SC, Canale RP (2008) Numerical methods for engineers. McGraw-Hill, New York
Chen SJ, Zhu M, Fu Y, Huang YX, Tao ZC, Li WL (2017) Using 13X, LiX, and LiPdAgX zeolites for CO2 capture from post-combustion flue gas. Appl Energy 191:87–98
Doelle HJ, Riekert L (1979) Kinetics of sorption, desorption, and diffusion in zeolites. Angew Chem Int Ed Eng 4:266–272
Doetschd IH, Ruthven DM, Loughlink F (1974) Sorption and diffusion of n-heptane in 5A zeolite. Can J Chem 52:2717–2724
Eagan JD, Kindl B, Anderson RB (1971) Kinetics of adsorption on A-zeolites -temperature effects. Adv Chem Ser 102:164
Garshasbi V, Jahangiri M, Anbia M (2017) Equilibrium CO2 adsorption on zeolite 13X prepared from natural clays. Appl Surf Sci 339:225–233
Glueckauf E (1955) Theory of chromatography. Part 10.—Formulæ for diffusion into spheres and their application to chromatography. J Chem Soc Faraday Soc 51:1540–1551
Hefti M, Marx D, Joss L, Mazzotti M (2015) Adsorption equilibrium of binary mixtures of carbon dioxide and nitrogen on zeolites ZSM-5 and 13X. Microporous Mesoporous Mater 215:215–228
Hosseini SF, Talaie MR, Aghamire S, Khademi MH, Gholami M, Esfahany MN (2017) Mathematical modeling of rapid temperature swing adsorption: the role of influencing parameters. Sep Purif Technol 183:181–193
Hsu LK, Haynes HW (1981) Effective diffusivity by the gas chromatography technique: analysis and application to measurements of diffusion of various hydrocarbons in zeolite NaY. AIChE J 27(1):81–91
Kiselev AV (1971) Vapor adsorption on zeolites considered as crystalline specific adsorbents. ACS Symp Ser 102:37
Langmuir I (1918) The Adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361
Lee L-K (1976) Ph.D. thesis, University of New Brunswick, Fredricton, Canada
Lee LK, Ruthven DM (1979) Analysis of thermal effects in adsorption rate measurements. J Chem Soc Faraday Trans 1 Phys Chem Condens Phases 75: 2406–2422
Luz AD, Guelli Ulson de Souza SMA, da Luz C, de Mello JMM, Ulson de Souza AA (2013a) Analysis of competition between multicomponent BTX compounds for the active site of adsorption in a fixed-bed column. Ind Eng Chem Res 52:16911–16921
Luz AD, Guelli Ulson de Souza SMA, da Luz C, Rezende RVdeP, Ulson de Souza AA (2013b) Multicomponent adsorption and desorption of BTX compounds using coconut shell activated carbon: experiments, mathematical modeling, and numerical simulation. Ind Eng Chem Res 52:7896–7911
Martinez G, Basmadjian D (1996) Towards a general gas adsorption isotherm. Chem Eng Sci 51:1043
McCabe WL, Julian CS, Peter H (1993) Unit operations of chemical engineering, vol 5. McGraw-Hill, New York
Nitta T, Shigetomi T, Kuro-Oka M, Katayama T (1984) An adsorption isotherm of multisite occupancy model for homogeneous surface. J Chem Eng Jpn 17:39
Rezaei F, Grahn M (2012) Thermal management of structured adsorbents in CO2 capture processes. Ind Eng Chem Res 51:4025–4034
Ruthven DM (1984) Principles of adsorption and adsorption process. Wiley, New York
Ruthven DM, Kaul BK (1996) Adsorption of n-hexane and intermediate molecular weight aromatic hydrocarbons on LaY zeolite. Ind Eng Chem Res 35:2060–2064
Ruthven D, Lee L, Yucel H (1980) Kinetics of non-isothermal sorption in molecular sieve crystals. AIChE J 26(1):16–23
Satterfield CN, Frahetti AJ (1967) Sorption and diffusion of gaseous hydrocarbons in synthetic mordenite. AIChE J 13:731
Satterfield CN, Katzer JR (1971) Counter-diffusion of liquid hydrocarbons in type Y zeolites. Ads Chem 102:193
Silva JA, Rodrigues AE (1997a) Sorption and diffusion on n-pentane in pellets of 5A Zeolite. Ind Eng Chem Res 36:493–500
Silva JA, Rodrigues AE (1997b) Equilibrium and kinetics n-hexane sorption in pellets of 5A Zeolite. AIChE J 43:2524–2534
Silva A, Mariani VC, Guelli Ulson de Souza AA, Guelli Ulson de Souza SMA (2005) Numerical study of n-pentane separation using adsorption column. Braz Arch Biol Technol 48:267–274
Sircar S, Myers AL (1985) Gas adsorption operations: equilibrium, kinetics, column dynamics and design. Adsorpt Sci Technol 2:69–87
Symoniak MF (1980) Upgrade naphtha to fuels and feedstocks. Hydroc Process 59(5):110–114
Tavolaro A, Drioli E (1999) Zeolite membranes. Adv Mater 11(12):975–996
Vavlitis AP, Ruthven DM, Loughlin KF (1981) Sorption of n-pentane, n-octane and n-decane in 5A zeolite crystals. J Colloid Interface Sci 84:526
Wakao N (1976) Particle-to-fluid transfer coefficients and fluid diffusivities at low flow rate in packed beds. Chem Eng Sci 31:1115–1122
Wakao N, Funazkri T (1978) Effect of fluid dispersion coefficients on particle-to-fluid mass transfer coefficients in packed beds: correlation of Sherwood numbers. Chem Eng Sci 33(10):1375–1384
Welty JR, Wicks CE, Wilson RE, Rorrer GL (2009) Fundamentals of momentum, heat, and mass transfer. Wiley, New York
Yang RT (1987) Gas separation by adsorption process. Butterworth, Stoneham
Youngquist GR, Allen JL, Eisenberg J (1971) Adsorption of hydrocarbons by synthetic zeolites. Ind Eng Chem Prod Res Dev 10(3):308–314
Zhang L, Qian G, Liu Z, Cui Q, Wang H, Yao H (2015) Adsorption and separation properties of n-pentane/isopentane on ZIF-8. Sep Purif Technol 156:472–479
Zhao Y, Liu X, Han Y (2015) Microporous carbonaceous adsorbents for CO2 separation via selective adsorption. RSC Adv 5:30310–30330
Zito PF, Caravella A, Brunetii A, Drioli E, Barbieri G (2017) Light gases saturation loading dependence on temperature in LTA 4A zeolite. Microporous Mesoporous Mater 249:67–77
Acknowledgements
The authors thank the Federal University of Fronteira Sul—UFFS, campus Erechim, and the Fundação Universidade do Estado de Santa Catarina—UDESC Oeste—SC, for the infrastructure yielded for the development of the research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Richit, L.A., Wolf, T.C., Ribeiro, M.C. et al. Finite difference approximation in a non-isothermal and non-adiabatic fixed bed adsorption model: an application to n-hexane. Braz. J. Chem. Eng. 37, 249–262 (2020). https://doi.org/10.1007/s43153-020-00015-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-020-00015-z