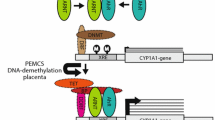
Abstract
Multiple environmental, behavioral, and hereditary factors affect pregnancy. Recent studies suggest that epigenetic modifications, such as DNA methylation (DNAm), affect both maternal and fetal health during the period of gestation. Some of the pregnancy-related risk factors can influence maternal DNAm, thus predisposing both the mother and the neonate to clinical adversities with long-lasting consequences. DNAm alterations in the promoter and enhancer regions modulate gene expression changes which play vital physiological role. In this review, we have discussed the recent advances in our understanding of maternal DNA methylation changes during pregnancy and its associated complications such as gestational diabetes and anemia, adverse pregnancy outcomes like preterm birth, and preeclampsia. We have also highlighted some major gaps and limitations in the area which if addressed might improve our understanding of pregnancy and its associated adverse clinical conditions, ultimately leading to healthy pregnancies and reduction of public health burden.
Similar content being viewed by others
References
Dadvand P, Parker J, Bell ML, Bonzini M, Brauer M, Darrow LA, et al. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ Health Perspect. 2013;121:267–373.
Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261:412–7.
Carmichael SL, Yang W, Shaw GM, others. Maternal dietary nutrient intake and risk of preterm delivery. Am J Perinatol. 2013;30:579–88.
Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, Gillman MW, Hennekens CH, et al. Birthweight and the risk for type 2 diabetes mellitus in adult women. Ann Intern Med. 1999;130:278–84.
Susser ES, Lin SP. Schizophrenia after prenatal exposure to the Dutch Hunger Winter of 1944-1945. Arch Gen Psychiatry. 1992;49:983–8.
Michels KB, Xue F. Role of birthweight in the etiology of breast cancer. Int J Cancer. 2006;119:2007–25.
Stuebe AM, Forman MR, Michels KB. Maternal-recalled gestational weight gain, pre-pregnancy body mass index, and obesity in the daughter. Int J Obes. 2009;33:743–52.
Johnson MP, Brennecke SP, East CE, et al. Genome-wide association scan identifies a risk locus for preeclampsia on 2q14, near the inhibin, beta b gene. PLoS One. 2012;7:e33666.
Hong X, Hao K, Ji H, Peng S, Sherwood B, di Narzo A, et al. Genome-wide approach identifies a novel gene-maternal pre-pregnancy bmi interaction on preterm birth. Nat Commun. 2017;8:15608.
Rappoport N, Toung J, Hadley D, Wong RJ, Fujioka K, Reuter J, et al. A genome-wide association study identifies only two ancestry specific variants associated with spontaneous preterm birth. Sci Rep. 2018;8:226.
Zhang YP, Liu XH, Gao SH, Wang JM, Gu YS, Zhang JY, et al. Risk factors for preterm birth in five maternal and child health hospitals in Beijing. PLoS One. 2012;7:e52780.
Nieuwenhuijsen MJ, Dadvand P, Grellier J, Martinez D, Vrijheid M. Environmental risk factors of pregnancy outcomes: a summary of recent meta-analyses of epidemiological studies. Environ Health. 2013;12:6.
Rogac M, Peterlin B. Epigenetic signature of chronic maternal stress load during pregnancy might be a potential biomarker for spontaneous preterm birth. Balkan J Med Genet. 2018;21:27–33.
Hoyo C, Murphy SK, Jirtle RL (2009) Imprint regulatory elements as epigenetic biosensors of exposure in epidemiological studies.
Relton CL, Smith GD. Epigenetic epidemiology of common complex disease: prospects for prediction, prevention, and treatment. PLoS Med. 2010;7:e1000356.
Dolinoy DC, Weidman JR, Jirtle RL. Epigenetic gene regulation: linking early developmental environment to adult disease. Reprod Toxicol. 2007;23:297–307.
Groom A, Elliott H, Embleton N, Relton C. Epigenetics and child health: basic principles. Arch Dis Child. 2011;96:863–9.
Cao-Lei L, Dancause KN, Elgbeili G, Laplante DP, Szyf M, King S. DNA methylation mediates the effect of maternal cognitive appraisal of a disaster in pregnancy on the child’s c-peptide secretion in adolescence: Project Ice Storm. PLoS One. 2018;13:e0192199.
Suter MA, Aagaard-Tillery KM. Environmental influences on epigenetic profiles. Semin Reprod Med. 2009;27:380–90.
Feinberg AP. Epigenetics at the epicenter of modern medicine. Jama. 2008;299:1345–50.
Talens RP, Boomsma DI, Tobi EW, Kremer D, Jukema JW, Willemsen G, et al. Variation, patterns, and temporal stability of DNA methylation: considerations for epigenetic epidemiology. FASEB J. 2010;24:3135–44.
Kim M, Costello J. DNA methylation: an epigenetic mark of cellular memory. Exp Mol Med. 2017;49:e322.
Duncan EJ, Gluckman PD, Dearden PK. Epigenetics, plasticity, and evolution: how do we link epigenetic change to phenotype? J Exp Zool B Mol Dev Evol. 2014;322:208–20.
McCarthy MI, Hirschhorn JN. Genome-wide association studies: potential next steps on a genetic journey. Hum Mol Genet. 2008;17:R156–65.
Maher B. Personal genomes: the case of the missing heritability. Nature News. 2008;456:18–21.
Huh SJ, Clement K, Jee D, Merlini A, Choudhury S, Maruyama R, et al. Age-and pregnancy-associated dna methylation changes in mammary epithelial cells. Stem cell reports. 2015;4:297–311.
Lumey L, Stein AD, Kahn HS, Van der Pal-de Bruin KM, Blauw G, Zybert PA, et al. Cohort profile: the Dutch Hunger Winter families study. Int J Epidemiol. 2007;36:1196–204.
Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci. 2008;105:17046–9.
Smith F, Garfield A, Ward A. Regulation of growth and metabolism by imprinted genes. Cytogenetic and genome research. 2006;113:279–91.
Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–62.
(2020) DNA methylation, an epigenetic mode of gene expression regulation in reproductive science. Harvard Catalyst Profiles Harvard Catalyst.
Bischof P. Trophoblast differentiation and invasion: its significance for human embryo implantation. Early Pregnancy. 1997;3:81.
Kim S-M, Kim J-S. A review of mechanisms of implantation. Development & reproduction. 2017;21:351–9.
Müller HM, Ivarsson L, Schröcksnadel H, et al. DNA methylation changes in sera of women in early pregnancy are similar to those in advanced breast cancer patients. Clin Chem. 2004;50:1065–8.
De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110.
Xiao F-H, Wang H-T, Kong Q-P (2019) Dynamic DNA methylation during aging: a “prophet” of age-related outcomes. Frontiers in genetics 10:
Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221:80–7.
Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev Immunol. 2013;31:387–411.
Svensson-Arvelund J, Ernerudh J, Buse E, Cline JM, Haeger J-D, Dixon D, Markert UR, Pfarrer C, Vos PD, Faas MM (2014) The placenta in toxicology. Part ii: systemic and local immune adaptations in pregnancy. Toxicologic pathology 42:327–338.
Romero R. Novel aspects of neutrophil biology in human pregnancy. Am J Reprod Immunol. 2005;53:275.
Petrušić V, Živković I, Muhandes L, Dimitrijević R, Stojanović M, Dimitrijević L. Infection-induced autoantibodies and pregnancy related pathology: an animal model. Reprod Fertil Dev. 2014;26:578–86.
Veenstra van Nieuwenhoven A, Heineman M, Faas M. The immunology of successful pregnancy. Hum Reprod Update. 2003;9:347–57.
White WM, Brost BC, Sun Z, Rose C, Craici I, Wagner SJ, et al. Normal early pregnancy: a transient state of epigenetic change favoring hypomethylation. Epigenetics. 2012;7:729–34.
Herrmann-Lavoie C, Rao C, Akoum A. Chorionic gonadotropin down-regulates the expression of the decoy inhibitory interleukin 1 receptor type ii in human endometrial epithelial cells. Endocrinology. 2007;148:5377–84.
Kuhajda FP, Piantadosi S, Pasternack GR. Haptoglobin-related protein (hpr) epitopes in breast cancer as a predictor of recurrence of the disease. N Engl J Med. 1989;321:636–41.
Hoffman L, Winfrey V, Blaeuer G, Olson G. A haptoglobin-like glycoprotein is produced by implantation-stage rabbit endometrium. Biol Reprod. 1996;55:176–84.
Wongpaiboonwattana W, Tosukhowong P, Dissayabutra T, Mutirangura A, Boonla C. Oxidative stress induces hypomethylation of line-1 and hypermethylation of the runx3 promoter in a bladder cancer cell line. Asian Pac J Cancer Prev. 2013;14:3773–8.
Burris HH, Rifas-Shiman SL, Baccarelli A, Tarantini L, Boeke CE, Kleinman K, et al. Associations of LINE-1 DNA methylation with preterm birth in a prospective cohort study. J Dev Orig Health Dis. 2012;3:173–81.
Gruzieva O, Merid SK, Chen S, et al. DnA methylation trajectories during pregnancy. Epigenetics insights. 2019;12:2516865719867090.
Poon LL, Leung TN, Lau TK, Chow KC, Lo YD. Differential DNA methylation between fetus and mother as a strategy for detecting fetal DNA in maternal plasma. Clin Chem. 2002;48:35–41.
Chim SS, Tong YK, Chiu RW, Lau TK, Leung TN, Chan LY, et al. Detection of the placental epigenetic signature of the maspin gene in maternal plasma. Proc Natl Acad Sci. 2005;102:14753–8.
Chiu RW, Chim SS, Wong IH, et al. Hypermethylation of rassf1a in human and rhesus placentas. Am J Pathol. 2007;170:941–50.
Ramo-Fernández L, Boeck C, Koenig AM, Schury K, Binder EB, Gündel H, et al. The effects of childhood maltreatment on epigenetic regulation of stress-response associated genes: an intergenerational approach. Sci Rep. 2019;9:1–12.
Bellido ML, Radpour R, Lapaire O, Bie ID, Hösli I, Bitzer J, et al. MALDI-tof mass array analysis of rassf1a and serpinb5 methylation patterns in human placenta and plasma. Biol Reprod. 2010;82:745–50.
Kulkarni A, Chavan-Gautam P, Mehendale S, Yadav H, Joshi S. Global DNA methylation patterns in placenta and its association with maternal hypertension in pre-eclampsia. DNA Cell Biol. 2011;30:79–84.
Zhao A, Cheng Y, Li X, Li Q, Wang L, Xu J, et al. Zhao X. Mol Hum Reprod. 2010;17:199–206.
Katari S, Turan N, Bibikova M, Erinle O, Chalian R, Foster M, et al. DNA methylation and gene expression differences in children conceived in vitro or in vivo. Hum Mol Genet. 2009;18:3769–78.
Bouchard L, Thibault S, Guay S-P, Santure M, Monpetit A, St-Pierre J, et al. Leptin gene epigenetic adaptation to impaired glucose metabolism during pregnancy. Diabetes Care. 2010;33:2436–41.
Velegrakis A, Sfakiotaki M, Sifakis S. Human placental growth hormone in normal and abnormal fetal growth. Biomed Rep. 2017;7:115–22.
Masuyama H, Hiramatsu Y. Potential role of estradiol and progesterone in insulin resistance through constitutive androstane receptor. J Mol Endocrinol. 2011;47:229–39.
de Barros MC, Lopes MA, Francisco RP, Sapienza AD, Zugaib M. Resistance exercise and glycemic control in women with gestational diabetes mellitus. Am J Obstet Gynecol. 2010;203:556–e1.
Marion D (2008) Screening and diagnosis of gestational diabetes mellitus. UpToDate web site.
Weng X, Liu F, Zhang H, Kan M, Wang T, Dong M, et al. Genome-wide DNA methylation profiling in infants born to gestational diabetes mellitus. Diabetes Res Clin Pract. 2018;142:10–8.
Enquobahrie DA, Moore A, Muhie S, Tadesse MG, Lin S, Williams MA. Early pregnancy maternal blood DNA methylation in repeat pregnancies and change in gestational diabetes mellitus status—a pilot study. Reprod Sci. 2015;22:904–10.
Loeffen J, Smeets R, Smeitink J, Triepels R, Sengers R, Trijbels F, et al. The human NADH: ubiquinone oxidoreductase ndufs5 (15kDa) subunit: CDNA cloning, chromosomal localization, tissue distribution and the absence of mutations in isolated complex i-deficient patients. J Inherit Metab Dis. 1999;22:19–28.
Patti M-E, Corvera S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev. 2010;31:364–95.
Qiu C, Enquobahrie DA, Frederick IO, Sorensen TK, Fernandez MAL, David RM, et al. Early pregnancy urinary biomarkers of fatty acid and carbohydrate metabolism in pregnancies complicated by gestational diabetes. Diabetes Res Clin Pract. 2014;104:393–400.
Kang J, Lee C-N, Li H-Y, Hsu K-H, Lin S-Y. Genome-wide DNA methylation variation in maternal and cord blood of gestational diabetes population. Diabetes Res Clin Pract. 2017;132:127–36.
Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr. 2000;71:1256S–61S.
Milano W, De Rosa M, Milano L, Capasso A. Antipsychotic drugs opposite to metabolic risk: neurotransmitters, neurohormonal and pharmacogenetic mechanisms underlying with weight gain and metabolic syndrome. The open neurology journal. 2013;7:23–31.
Wendland EM, Torloni MR, Falavigna M, Trujillo J, Dode MA, Campos MA, et al. Gestational diabetes and pregnancy outcomes–a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth. 2012;12:23.
Ruchat S-M, Houde A-A, Voisin G, St-Pierre J, Perron P, Baillargeon J-P, et al. Gestational diabetes mellitus epigenetically affects genes predominantly involved in metabolic diseases. Epigenetics. 2013;8:935–43.
Finer S, Mathews C, Lowe R, Smart M, Hillman S, Foo L, et al. Maternal gestational diabetes is associated with genome-wide DNA methylation variation in placenta and cord blood of exposed offspring. Hum Mol Genet. 2015;24:3021–9.
Baron Gaillard CL, Pallesi-Pocachard E, Massey-Harroche D, Richard F, Arsanto J-P, Chauvin J-P, et al. Hook2 is involved in the morphogenesis of the primary cilium. Mol Biol Cell. 2011;22:4549–62.
Haeseleer F, Jang G-F, Imanishi Y, Driessen CA, Matsumura M, Nelson PS, et al. Dual-substrate specificity short chain retinol dehydrogenases from the vertebrate retina. J Biol Chem. 2002;277:45537–46.
Wu P, Farrell WE, Haworth KE, Emes RD, Kitchen MO, Glossop JR, et al. Maternal genome-wide DNA methylation profiling in gestational diabetes shows distinctive disease-associated changes relative to matched healthy pregnancies. Epigenetics. 2018;13:122–8.
De Benoist B, Cogswell M, Egli I, McLean E (2008) Worldwide prevalence of anaemia 1993-2005; who global database of anaemia.
Greenberg JA, Bell SJ, Guan Y, Yu Y-h. Folic acid supplementation and pregnancy: more than just neural tube defect prevention. Rev Obstet Gynecol. 2011;4:52.
Pritchard JA, Adams RH. Erythrocyte production and destruction during pregnancy. American Journal of Obstetrics & Gynecology. 1960;79:750–7.
Sharma JB, Shankar M. Anemia in pregnancy. JIMSA. 2010;23:253–60.
Tamura T, Picciano MF. Folate and human reproduction. Am J Clin Nutr. 2006;83:993–1016.
Joubert BR, Herman T, Felix JF, et al. Maternal plasma folate impacts differential DNA methylation in an epigenome-wide meta-analysis of newborns. Nat Commun. 2016;7:10577.
Knight AK, Park HJ, Hausman DB, Fleming JM, Bland VL, Rosa G, et al. Association between one-carbon metabolism indices and DNA methylation status in maternal and cord blood. Sci Rep. 2018;8:16873.
Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller A-B, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10:S2.
Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the sustainable development goals. Lancet. 2016;388:3027–35.
Menon R. Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet Gynecol Scand. 2008;87:590–600.
Frey HA, Stout MJ, Pearson LN, Tuuli MG, Cahill AG, Strauss JF III, et al. Genetic variation associated with preterm birth in African-American women. Am J Obstet Gynecol. 2016;215:235–e1.
Menon R, Fortunato SJ, Velez Edwards DR, Williams SM. Association of genetic variants, ethnicity and preterm birth with amniotic fluid cytokine concentrations. Ann Hum Genet. 2010;74:165–83.
Zhang G, Feenstra B, Bacelis J, Liu X, Muglia LM, Juodakis J, et al. Genetic associations with gestational duration and spontaneous preterm birth. N Engl J Med. 2017;377:1156–67.
Hong X, Sherwood B, Ladd-Acosta C, Peng S, Ji H, Hao K, et al. Genome-wide DNA methylation associations with spontaneous preterm birth in US blacks: findings in maternal and cord blood samples. Epigenetics. 2018;13:163–72.
Chen Q, Coffey A, Bourgoin SG, Gadina M. Cytohesin binder and regulator augments t cell receptor-induced nuclear factor of activated t cells ap-1 activation through regulation of the jnk pathway. J Biol Chem. 2006;281:19985–94.
O’Brien M, Morrison JJ, Smith TJ. Upregulation of pscdbp, tlr2, twist1, flj35382, ednrb, and rgs12 gene expression in human myometrium at labor. Reprod Sci. 2008;15:382–93.
Kropf P, Baud D, Marshall SE, et al. Arginase activity mediates reversible T cell hyporesponsiveness in human pregnancy. Eur J Immunol. 2007;37:935–45.
Jeanty C, Derderian SC, Mackenzie TC. Maternal-fetal cellular trafficking: clinical implications and consequences. Curr Opin Pediatr. 2014;26:377–82.
Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23:1494–504.
Consortium F, II Team RGERGPI &, others (2002) Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature 420:563, 573.
Chen SJ, Liu YL, Sytwu HK. Immunologic regulation in pregnancy: from mechanism to therapeutic strategy for immunomodulation. Clin Dev Immunol. 2012;2012:258391.
Parets SE, Conneely KN, Kilaru V, Menon R, Smith AK. DNA methylation provides insight into intergenerational risk for preterm birth in African Americans. Epigenetics. 2015;10:784–92.
Foster HA, Davies J, Pink RC, Turkcigdem S, Goumenou A, Carter DR, et al. The human myometrium differentially expresses mTOR signalling components before and during pregnancy: evidence for regulation by progesterone. J Steroid Biochem Mol Biol. 2014;139:166–72.
Laplante M, Sabatini DM. MTOR signaling at a glance. J Cell Sci. 2009;122:3589–94.
Jaffer S, Shynlova O, Lye S. Mammalian target of rapamycin is activated in association with myometrial proliferation during pregnancy. Endocrinology. 2009;150:4672–80.
McRae AF, Powell JE, Henders AK, Bowdler L, Hemani G, Shah S, et al. Contribution of genetic variation to transgenerational inheritance of DNA methylation. Genome Biol. 2014;15:R73.
Fujimoto T, Parry S, Urbanek M, Sammel M, Macones G, Kuivaniemi H, et al. A single nucleotide polymorphism in the matrix metalloproteinase-1 (MMP-1) promoter influences amnion cell MMP-1 expression and risk for preterm premature rupture of the fetal membranes. J Biol Chem. 2002;277:6296–302.
Wang H, Ogawa M, Wood JR, Bartolomei MS, Sammel MD, Kusanovic JP, et al. Genetic and epigenetic mechanisms combine to control mmp1 expression and its association with preterm premature rupture of membranes. Hum Mol Genet. 2008;17:1087–96.
Moutquin J-M. Classification and heterogeneity of preterm birth. BJOG Int J Obstet Gynaecol. 2003;110:30–3.
Knijnenburg TA, Vockley JG, Chambwe N, Gibbs DL, Humphries C, Huddleston KC, et al. Genomic and molecular characterization of preterm birth. Proc Natl Acad Sci. 2019;116:5819–27.
Winn VD, Haimov-Kochman R, Paquet AC, Yang YJ, Madhusudhan MS, Gormley M, et al. Gene expression profiling of the human maternal-fetal interface reveals dramatic changes between midgestation and term. Endocrinology. 2007;148:1059–79.
Denis M, Enquobahrie DA, Tadesse MG, Gelaye B, Sanchez SE, Salazar M, et al. Placental genome and maternal-placental genetic interactions: a genome-wide and candidate gene association study of placental abruption. PLoS One. 2014;9:e116346.
Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, et al. Inducible gene knockout of transcription factor recombination signal binding protein-j reveals its essential role in t versus b lineage decision. Int Immunol. 2002;14:637–45.
Cunningham FG, Leveno K, Bloom S, Hauth J, Gilstrap L, Wenstrom K (2005) Gestational trophoblastic disease. Williams Obstetrics. 22nd ed. New York: McGraw Hill.
Small MJ, Kershaw T, Frederic R, Blanc C, Neale D, Copel J, et al. Characteristics of preeclampsia-and eclampsia-related maternal death in rural Haiti. J Matern Fetal Neonatal Med. 2005;18:343–8.
Manandhar T, Prashad B, Nath Pal M. Risk factors for intrauterine growth restriction and its neonatal outcome. Gynecol Obstet. 2018;8:2161–0932.
Walsh SW. Obesity: a risk factor for preeclampsia. Trends in Endocrinology & Metabolism. 2007;18:365–70.
Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11:342–52.
Hing B, Braun P, Cordner ZA, Ewald ER, Moody L, McKane M, et al. Chronic social stress induces DNA methylation changes at an evolutionary conserved intergenic region in chromosome X. Epigenetics. 2018;13:627–41.
He F, Berg A, Kawasawa YI, Bixler EO, Fernandez-Mendoza J, Whitsel EA, et al. Association between DNA methylation in obesity-related genes and body mass index percentile in adolescents. Sci Rep. 2019;9:2079.
Wilson SL, Leavey K, Cox B, Robinson WP. The value of DNA methylation profiling in characterizing preeclampsia and intrauterine growth restriction. BioRxiv. 2017;151290.
Leavey K, Wilson SL, Bainbridge SA, Robinson WP, Cox BJ. Epigenetic regulation of placental gene expression in transcriptional subtypes of preeclampsia. Clin Epigenetics. 2018;10:28.
Wilson SL, Leavey K, Cox BJ, Robinson WP. Mining DNA methylation alterations towards a classification of placental pathologies. Hum Mol Genet. 2018;27:135–46.
Tsui DW, Chan KA, Chim SS, L-w C, T-y L, T-k L, et al. Quantitative aberrations of hypermethylated rassf1a gene sequences in maternal plasma in pre-eclampsia. Prenatal Diagnosis: Published in Affiliation With the International Society for Prenatal Diagnosis. 2007;27:1212–8.
Bianchi DW (2004) Circulating fetal DNA: its origin and diagnostic potential-a review. Placenta 25 Suppl A:S93–S101.
Emlen W, Mannik M. Effect of DNA size and strandedness on the in vivo clearance and organ localization of DNA. Clin Exp Immunol. 1984;56:185–92.
TSUMITA T, IWANAGA M. Fate of injected deoxyribonucleic acid in mice. Nature. 1963;198:1088–9.
Hesson LB, Cooper WN, Latif F. The role of RASSF1A methylation in cancer. Dis Markers. 2007;23:73–87.
Chiu RW, Chim SS, Wong IH, et al. Hypermethylation of RASSF1A in human and rhesus placentas. Am J Pathol. 2007;170:941–50.
Lee SB, Wong AP, Kanasaki K, Xu Y, Shenoy VK, McElrath TF, et al. Preeclampsia: 2-methoxyestradiol induces cytotrophoblast invasion and vascular development specifically under hypoxic conditions. Am J Pathol. 2010;176:710–20.
Mousa AA, Archer KJ, Cappello R, Estrada-Gutierrez G, Isaacs CR, Strauss JF III, et al. DNA methylation is altered in maternal blood vessels of women with preeclampsia. Reprod Sci. 2012;19:1332–42.
Tanabe T, Ullrich V. Prostacyclin and thromboxane synthases. J Lipid Mediat Cell Signal. 1995;12:243–55.
Walsh SW. Preeclampsia: an imbalance in placental prostacyclin and thromboxane production. Am J Obstet Gynecol. 1985;152:335–40.
Chavarrı́a ME, Lara-González L, González-Gleason A, Garcı́a-Paleta Y, Vital-Reyes VS, Reyes A (2003) Prostacyclin/thromboxane early changes in pregnancies that are complicated by preeclampsia. Am J Obstet Gynecol 188:986–992.
Anderson CM, Ralph JL, Wright ML, Linggi B, Ohm JE. DNA methylation as a biomarker for preeclampsia. Biological research for nursing. 2014;16:409–20.
Schulz LC, Widmaier EP, Qiu J, Roberts RM. Effect of leptin on mouse trophoblast giant cells. Biol Reprod. 2009;80:415–24.
Liu AX, Jin F, Zhang WW, Zhou TH, Zhou CY, Yao WM, et al. Proteomic analysis on the alteration of protein expression in the placental villous tissue of early pregnancy loss. Biol Reprod. 2006;75:414–20.
Abumaree MH, Chamley LW, Badri M, El-Muzaini MF. Trophoblast debris modulates the expression of immune proteins in macrophages: a key to maternal tolerance of the fetal allograft? J Reprod Immunol. 2012;94:131–41.
Liu B, Xu Y, Voss C, Qiu FH, Zhao MZ, Liu YD, et al. Altered protein expression in gestational diabetes mellitus placentas provides insight into insulin resistance and coagulation/fibrinolysis pathways. PLoS One. 2012;7:e44701.
Sharma D, Shastri S, Sharma P (2016) Intrauterine growth restriction: antenatal and postnatal aspects. Clinical Medicine Insights: Pediatrics 10:CMPed–S40070.
Martin EM, Fry RC. Environmental influences on the epigenome: exposure-associated DNA methylation in human populations. Annu Rev Public Health. 2018;39:309–33.
Koh W, Pan W, Gawad C, Fan HC, Kerchner GA, Wyss-Coray T, et al. Noninvasive in vivo monitoring of tissue-specific global gene expression in humans. Proc Natl Acad Sci U S A. 2014;111:7361–6.
Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC bioinformatics. 2012;13:86.
Rahmani E, Schweiger R, Shenhav L, Wingert T, Hofer I, Gabel E, et al. BayesCCE: a Bayesian framework for estimating cell-type composition from dna methylation without the need for methylation reference. Genome Biol. 2018;19:1–18.
Li Z, Wu H. TOAST: improving reference-free cell composition estimation by cross-cell type differential analysis. Genome Biol. 2019;20:190.
Michalczyk AA, Janus ED, Judge A, Ebeling PR, Best JD, Ackland MJ, et al. Transient epigenomic changes during pregnancy and early postpartum in women with and without type 2 diabetes. Epigenomics. 2018;10:419.
Moen G-H, Sommer C, Prasad RB, Sletner L, Groop L, Qvigstad E, et al. MECHANISMS IN ENDOCRINOLOGY: epigenetic modifications and gestational diabetes: a systematic review of published literature. Eur J Endocrinol. 2017;176:R247–67.
Jia N, Li J. Noncoding RNAs in unexplained recurrent spontaneous abortions and their diagnostic potential. Dis Markers. 2019. https://doi.org/10.1155/2019/7090767.
Wang G, Divall S, Radovick S, Paige D, Ning Y, Chen Z, et al. Preterm birth and random plasma insulin levels at birth and in early childhood. Jama. 2014;311:587–96.
Parets SE, Knight AK, Smith AK. Insights into genetic susceptibility in the etiology of spontaneous preterm birth. Appl Clin Genet. 2015;8:283.
Bhatnagar S, Majumder PP, Salunke DM. A pregnancy cohort to study multidimensional correlates of preterm birth in India: study design, implementation, and baseline characteristics of the participants. Am J Epidemiol. 2019;188:621–31.
Acknowledgments
We thank Professor Partha Pratim Majumder for his mentorship, advice and suggestions.
Funding
JD is supported by the Research Fellowship (NET) of the University Grants Commission (UGC), India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Consents
This is a review article and does not involve collection of human biospecimens or analysis. The review is based on published information. Hence no ethical consent is required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Das, J., Maitra, A. Maternal DNA Methylation During Pregnancy: a Review. Reprod. Sci. 28, 2758–2769 (2021). https://doi.org/10.1007/s43032-020-00456-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00456-4