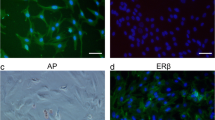
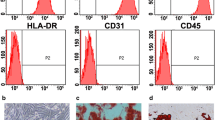
Abstract
Several lines of evidence strongly suggest that retinoic acid (RA) and stem cell factor (SCF)/c-Kit signal transduction pathways are involved in the differentiation of spermatogonial stem cells (SSCs). This study was aimed to investigate the effect of RA and SCF on in vitro differentiation of SSCs via evaluation of the mRNA expression of meiosis-specific genes in cultured testicular tissues. Testicular tissue samples were obtained from bilaterally vasectomized rats and also healthy adult rats and then were cultured for 25, 30, and 35 days on different conditions. The cultured testicular pieces were sectioned and stained with PAS to histological analysis. The total RNA was extracted from cultured testicular samples, and the expression of ACR, PRTM1, SYCP3, STRA8, c-KIT, PIWIL2, and OCT4 genes at mRNA level was quantified using real-time polymerase chain reaction (qPCR) procedure. After 1-month surgery, bilateral testicular weight showed a significant decrease in vasectomized adult rats compared with healthy adult rats (P < 0.05). Reduction in the diameter of the seminiferous tubules and depletion of advanced germinal elements in vasectomized rats compared with healthy adult rats were also observed. Our findings also demonstrated that the mRNA expression level of PRTM1, STRA8, c-KIT, PIWIL2, and OCT4 genes in cultured testicular tissues significantly up-regulated in experimental group II compared with the control group (P < 0.001). Our findings lead us to conclude that SCF improves in vitro differentiation of SSCs in the OA rats, at least partially, by transcriptionally upregulating PRTM1, STRA8, c-KIT, PIWIL2, and OCT4 genes.





Similar content being viewed by others
Change history
22 February 2021
A Correction to this paper has been published: https://doi.org/10.1007/s43032-021-00498-2
References
Krausz C, Riera-Escamilla A. Genetics of male infertility. Nat Rev Urol. 2018;15:369–84.
Aston KI. Genetic susceptibility to male infertility: news from genome-wide association studies. Andrology. 2014;2:315–21.
Miyamoto T, Minase G, Shin T, Ueda H, Okada H, Sengoku K. Human male infertility and its genetic causes. Reprod Med Biol. 2017;16:81–8.
Leaver RB. Male infertility: an overview of causes and treatment options. Br J Nurs. 2016;25:35–40.
Lotti F, Maggi M. Sexual dysfunction and male infertility. Nat Rev Urol. 2018;15:287–307.
Bracke A, Peeters K, Punjabi U, Hoogewijs D, Dewilde S. A search for molecular mechanisms underlying male idiopathic infertility. Reprod BioMed Online. 2018;36:327–39.
Oatley JM, Brinster RL. Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol. 2008;24:263–86.
Griswold MD. Spermatogenesis: the commitment to meiosis. Physiol Rev. 2016;96:1–17.
Mecklenburg JM, Hermann BP. Mechanisms regulating spermatogonial differentiation. Results Probl Cell Differ. 2016;58:253–87.
de Rooij DG. Proliferation and differentiation of spermatogonial stem cells. Reproduction. 2001;121:347–54.
Sun M, Yuan Q, Niu M, Wang H, Wen L, Yao C, et al. Efficient generation of functional haploid spermatids from human germline stem cells by three-dimensional-induced system. Cell Death Differ. 2018;25:749–66.
Song HW, Wilkinson MF. In vitro spermatogenesis: a long journey to get tails. Spermatogenesis. 2012;2:238–44.
Ibtisham F, Wu J, Xiao M, An L, Banker Z, Nawab A, et al. Progress and future prospect of in vitro spermatogenesis. Oncotarget. 2017;8:66709–27.
Sato T, Katagiri K, Yokonishi T, Kubota Y, Inoue K, Ogonuki N, et al. In vitro production of fertile sperm from murine spermatogonial stem cell lines. Nat Commun. 2011;2:472.
Sato T, Katagiri K, Kubota Y, Ogawa T. In vitro sperm production from mouse spermatogonial stem cell lines using an organ culture method. Nat Protoc. 2013;8:2098–104.
Sato T, Katagiri K, Kojima K, Komeya M, Yao M, Ogawa T. In vitro spermatogenesis in explanted adult mouse testis tissues. PLoS One. 2015;10:e0130171.
Reda A, Hou M, Winton TR, Chapin RE, Söder O, Stukenborg JB. In vitro differentiation of rat spermatogonia into round spermatids in tissue culture. Mol Hum Reprod. 2016;22:601–12.
Gohbara A, Katagiri K, Sato T, Kubota Y, Kagechika H, Araki Y, et al. In vitro murine spermatogenesis in an organ culture system. Biol Reprod. 2010;83:261–7.
Sanjo H, Komeya M, Sato T, Abe T, Katagiri K, Yamanaka H, et al. In vitro mouse spermatogenesis with an organ culture method in chemically defined medium. PLoS One. 2018;13:e0192884.
Mohammadzadeh E, Mirzapour T, Nowroozi MR, Nazarian H, Piryaei A, Alipour F, et al. Differentiation of spermatogonial stem cells by soft agar three-dimensional culture system. Artif Cells Nanomed Biotechnol. 2019;47:1772–81.
Ziloochi Kashani M, Bagher Z, Asgari HR, Najafi M, Koruji M, Mehraein F. Differentiation of neonate mouse spermatogonial stem cells on three-dimensional agar/polyvinyl alcohol nanofiber scaffold. Syst Biol Reprod Med. 2020;6(1):1–14.
AbuMadighem A, Solomon R, Stepanovsky A, Kapelushnik J, Shi Q, Meese E, et al. Development of spermatogenesis in vitro in three-dimensional culture from spermatogonial cells of busulfan-treated immature mice. Int J Mol Sci. 2018;19:E3804.
Roser JF. Regulation of testicular function in the stallion: an intricate network of endocrine, paracrine and autocrine systems. Anim Reprod Sci. 2008;107:179–96.
Huleihel M, Lunenfeld E. Regulation of spermatogenesis by paracrine/autocrine testicular factors. Asian J Androl. 2004;6:259–68.
Li X, Long XY, Xie YJ, Zeng X, Chen X, Mo ZC. The roles of retinoic acid in the differentiation of spermatogonia and spermatogenic disorders. Clin Chim Acta. 2019;497:54–60.
Busada JT, Geyer CB. The role of retinoic acid (RA) in spermatogonial differentiation. Biol Reprod. 2016;94:10.
Feng LX, Ravindranath N, Dym M. Stem cell factor/c-kit up-regulates cyclin D3 and promotes cell cycle progression via the phosphoinositide 3-kinase/p70 S6 kinase pathway in spermatogonia. J Biol Chem. 2000;275:25572–6.
Koli S, Mukherjee A, Reddy KVR. Retinoic acid triggers c-kit gene expression in spermatogonial stem cells through an enhanceosome constituted between transcription factor binding sites for retinoic acid response element (RARE), spleen focus forming virus proviral integration oncogene (SPFI1) (PU.1) and E26 transformation-specific (ETS). Reprod Fertil Dev. 2017;29:521–43.
Amann PM, Eichmüller SB, Schmidt J, Bazhin AV. Regulation of gene expression by retinoids. Curr Med Chem. 2011;18:1405–12.
Ijiri TW, Mahbub Hasan AK, Sato K. Protein-tyrosine kinase signaling in the biological functions associated with sperm. J Signal Transduct. 2012;2012:181560.
Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, et al. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471:504–7.
Russell LD, Ettlin RA, Hikim APS, Clegg ED. Histological and histopathological evaluation of the testis. Int J Androl. 1993;16:83.
Travers A, Arkoun B, Safsaf A, Milazzo JP, Absyte A, Bironneau A, et al. Effects of vitamin a on in vitro maturation of pre-pubertal mouse spermatogonial stem cells. PLoS One. 2013;8:e82819.
Arkoun B, Dumont L, Milazzo JP, Rondanino C, Bironneau A, Wils J, et al. Does soaking temperature during controlled slow freezing of pre-pubertal mouse testes influence course of in vitro spermatogenesis? Cell Tissue Res. 2016;364:661–74.
Dumont L, Oblette A, Rondanino C, Jumeau F, Bironneau A, Liot D, et al. Vitamin a prevents round spermatid nuclear damage and promotes the production of motile sperm during in vitro maturation of vitrified pre-pubertal mouse testicular tissue. Mol Hum Reprod. 2016;22:819–32.
Di Lorenzo A, Bedford MT. Histone arginine methylation. FEBS Lett. 2011;585:2024–31.
Li J, Zhou F, Zhan D, Gao Q, Cui N, Li J, et al. A novel histone H4 arginine 3 methylation-sensitive histone H4 binding activity and transcriptional regulatory function for signal recognition particle subunits SRP68 and SRP72. J Biol Chem. 2012;287:40641–51.
Jeong HS, Bhin J, Joon Kim H, Hwang D, Ryul Lee D, Kim KS. Transcriptional regulatory networks underlying the reprogramming of spermatogonial stem cells to multipotent stem cells. Exp Mol Med. 2017;49:e315.
Ma HT, Niu CM, Xia J, Shen XY, Xia MM, Hu YQ, et al. Stimulated by retinoic acid gene 8 (Stra8) plays important roles in many stages of spermatogenesis. Asian J Androl. 2018;20:479–87.
Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, et al. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci U S A. 2008;105:14976–80.
Raverdeau M, Gely-Pernot A, Féret B, Dennefeld C, Benoit G, Davidson I, et al. Retinoic acid induces Sertoli cell paracrine signals for spermatogonia differentiation but cell autonomously drives spermatocyte meiosis. Proc Natl Acad Sci U S A. 2012;109:16582–7.
Zhou Q, Nie R, Li Y, Friel P, Mitchell D, Hess RA, et al. Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: an in vivo study in vitamin A-sufficient postnatal murine testes. Biol Reprod. 2008;79:35–42.
Cardoso HJ, Figueira MI, Socorro S. The stem cell factor (SCF)/c-KIT signalling in testis and prostate cancer. J Cell Commun Signal. 2017;11:297–307.
Zhang L, Tang J, Haines CJ, Feng H, Lai L, Teng X, et al. c-kit expression profile and regulatory factors during spermatogonial stem cell differentiation. BMC Dev Biol. 2013;13:38.
Lee JH, Engel W, Nayernia K. Stem cell protein Piwil2 modulates expression of murine spermatogonial stem cell expressed genes. Mol Reprod Dev. 2006;73:173–9.
Esch D, Vahokoski J, Groves MR, Pogenberg V, Cojocaru V, Vom Bruch H, et al. A unique Oct4 interface is crucial for reprogramming to pluripotency. Nat Cell Biol. 2013;15:295–301.
Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–6.
Dann CT, Alvarado AL, Molyneux LA, Denard BS, Garbers DL, Porteus MH. Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells. 2008;26:2928–37.
Acknowledgments
The authors would like to express their sincere gratitude to Vice-Chancellor of Research and Technology at Babol University of Medical Sciences, Cellular and Molecular Biology Research Center at Babol University of Medical Sciences, the staff of Stem Cell Laboratory of the Reproductive Health and Infertility Research Center of Fatemeh Al-Zahra Hospital and Islamic Azad University of Ayatollah Amoli.
Author information
Authors and Affiliations
Contributions
Mahnaz Nasimi, Seyed Gholam Ali Jorsaraei, and Esmail Fattahi designed the experiments, performed the statistical analysis, contributed to the interpretation of the results, and wrote the manuscript; Mahnaz Nasimi, Maryam Gholamitabar Tabari, and Ebrahim Zabihi Neyshaburi carried out the experiments; Seyed Gholam Ali Jorsaraei and Esmail Fattahi supervised the project.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare they have no potential conflicting financial interests.
Ethical Consents
The current study was approved by the ethics committee for experimental research on animals at Babol University of Medical Sciences, Babol, Iran.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article was updated to correct the spelling of Esmail Fattahi’s name in the author listing.
Rights and permissions
About this article
Cite this article
Nasimi, M., Jorsaraei, S.G.A., Fattahi, E. et al. SCF Improves In Vitro Differentiation of SSCs Through Transcriptionally Up-regulating PRTM1, STRA8, c-KIT, PIWIL2, and OCT4 Genes. Reprod. Sci. 28, 963–972 (2021). https://doi.org/10.1007/s43032-020-00326-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00326-z