Abstract
Inadequate nutrition and lifestyle behaviors, particularly during the periconception period, are associated with a negative impact on embryonic and subsequent fetal development. We investigated the associations between parental nutritional and lifestyle factors and pre-implantation embryo development. A total of 113 women and 41 partners, with a corresponding 490 embryos, who underwent intracytoplasmic sperm injection (ICSI) treatment subscribed to the mHealth coaching platform “Smarter Pregnancy.” At baseline, nutrition and lifestyle behaviors (intake of fruits, vegetables, folic acid, and smoking and alcohol use) were identified and risk scores were calculated. A lower risk score represents healthier behavior. As outcome measure, a time-lapse morphokinetic selection algorithm (KIDScore) was used to rank pre-implantation embryo quality on a scale from 1 (poor) to 5 (good) after being cultured in the Embryoscope™ time-lapse incubator until embryonic day 3. To study the association between the nutritional and lifestyle risk scores and the KIDScore in men and women, we used a proportional odds model. In women, the dietary risk score (DRS), a combination of the risk score of fruits, vegetables, and folic acid, was negatively associated with the KIDScore (OR 0.86 (95% CI 0.76 to 0.98), p = 0.02). This could mainly be attributed to an inadequate vegetable intake (OR 0.76 (95% CI 0.59 to 0.96), p = 0.02). In men, smoking was negatively associated with the KIDscore (OR 0.53 (95% CI 0.33 to 0.85), p < 0.01). We conclude that inadequate periconceptional maternal vegetable intake and paternal smoking significantly reduce the implantation potential of embryos after ICSI treatment. Identifying modifiable lifestyle risk factors can contribute to directed, personalized, and individual recommendations that can potentially increase the chance of a healthy pregnancy.
Similar content being viewed by others

Introduction
Subfertility is still an increasing problem in the Western world, which can be attributed to postponing pregnancy, but also to a decline in sperm count, increasing age of women at the time of conception, obesity, smoking, and other poor lifestyles [1,2,3]. Despite novel developments in assisted reproductive technology (ART), high rates of implantation failure and early pregnancy loss are still seen after the transfer of selected, morphologically high quality, pre-implantation embryos [4].
Pre-implantation embryo development can be studied using the EmbryoScope™, which incorporates a specialized built-in microscope designed for automated time-lapse embryo assessment by acquiring images [5]. The EmbryoScope™ provides a controlled culture environment and captures comprehensive information on embryo development without the need for handling or disturbing the developing embryo. The use of embryo morphokinetics by timing of embryo developmental events, available through continuous time-lapse monitoring, has added another dimension to current traditional morphology classification scores used to predict embryo implantation potential and viability [6, 7]. Animal studies have shown that single embryo developmental kinetics at the cleavage stage are reflective of culture conditions, but also of embryo metabolism, genetic integrity, and blastocyst formation and quality [8,9,10,11]. To assist in embryo selection as part of IVF treatment, the KIDScore algorithm was developed as a generally applicable morphokinetic algorithm suitable to rank day 3 embryos, originating from different culture conditions and fertilization methods. Embryos are ranked in five groups predicting their ability to develop into a blastocyst with an area under the curve (AUC) of 0.75 and implantation potential with an AUC of 0.65 (indicative of intermediate prediction) [12]. Interestingly, a recent study showed that the KIDScore was superior regarding predicting implantation and ongoing pregnancy rates when compared to only scoring embryo morphology [13].
Most reproductive challenges, such as fertility problems, miscarriages, congenital malformations, and fetal growth restriction, largely originate in the periconceptional period, which ranges from at least 14 weeks before conception until 10 weeks after conception [14,15,16]. Inadequate nutrition and lifestyle behaviors particularly during the periconception period are associated with a negative impact on the development of the embryo and subsequent fetal development [17]. Nutrition and lifestyle behaviors are specifically of clinical interest since these factors are modifiable. Couples contemplating pregnancy are often not aware of their inadequate nutrition and lifestyle behaviors and the detrimental effects on reproduction [18]. To investigate the effect of adherence to general dietary recommendations in couples undergoing IVF/ICSI treatment, our group studied the association with the chance of ongoing pregnancy. Improvement of adherence to the nutritional recommendations of the Dutch Nutrition Centre (covering the intake of six main food groups namely fruits, vegetables, meat, fish, whole wheat products, and fats) resulted in a 65% increase of ongoing pregnancy after IVF-ICSI treatment [19]. Furthermore, inadequate nutritional behaviors of the mother during pregnancy can also have detrimental consequences for the health of the offspring later in life, where earlier age of puberty-onset and a decline in ovarian follicle reserve have been reported [20]. Furthermore paternal obesity and nutritional factors are also linked to sperm quality and epigenetic profiles, possibly also affecting embryo quality and pregnancy outcomes [21, 22]. We hypothesize that paternal nutrition and lifestyle factors affect multiple pathways involved in the (patho)physiology of sperm quality, such as inflammation, vascular pathways, and epigenetics, that can also influence the development of pre-implantation embryos.
Nutrition and lifestyle behaviors can be easily assessed using the online mHealth program Smarter Pregnancy, which effectively improves the intake of fruits, vegetables, and folic acid supplements and stop smoking and use of alcoholic drinks [23]. The effect of nutrition and lifestyle behaviors on fertility and pregnancy outcomes are widely studied; however, their influence on pre-implantation embryo development is limited. Investigating pre-implantation embryo parameters in vitro provides a unique insight into the direct impact of maternal and paternal factors through oocyte and sperm, respectively, independent of the in vivo utero maternal environment. Therefore, the aim of this study is to investigate the associations between the five Smarter Pregnancy lifestyle behaviors (vegetables, fruit, folic acid, smoking, and alcohol use) of both men and women and the quality of development of pre-implantation embryos cultured in the EmbryoScope™ as a marker of implantation potential and assessed by the KIDScore algorithm.
Materials and Methods
Study Design, Population, and Patient Inclusion
In a prospective cohort study, couples that underwent ICSI treatment were included when embryos were cultured in the EmbryoScope™ time-lapse incubator and baseline data on the five nutrition and lifestyle behaviors of the mHealth program Smarter Pregnancy were available. Couples were included between October 2014 until December 2017 at the Erasmus MC, University Medical Center, Rotterdam, the Netherlands [23].
Patients had to be at least 18 years of age and had to have a good understanding of Dutch speaking and writing. After the introduction of the EmbryoScope™ time-lapse incubator in our clinic, it was mostly used for cycles from patients undergoing ICSI treatment, as in this situation, oocytes can be submitted to time-lapse culture directly after injection and resulting embryos can benefit optimally from undisturbed culture. Only in the more recent years, we also cultured some embryos from IVF treatment cycles in the EmbryoScope™. In this case, fertilized oocytes are submitted to time-lapse culture the day after insemination, after pronuclear inspection. In these cases, the time point of fertilization is less accurate than after ICSI and pronuclear appearance cannot be assessed. We therefore excluded these cycles for the current analysis. Furthermore, we excluded patients with no available data of the Smarter Pregnancy program.
Ethical Approval
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving patients were approved by the Medical Ethical Institutional Review Board of the Erasmus, University Medical Center, Rotterdam, the Netherlands. Written informed consent was obtained from all female and male participants at enrolment.
In Vitro Fertilization Procedures
Ovarian stimulation, oocyte retrieval, the ICSI procedures, and assessment of embryo morphology were performed as described previously [24]. Inseminated oocytes were cultured in the EmbryoScope™ in Sage 1-step culture medium (Origio/Cooper Surgical™, Denmark) at 36.8 °C, 7% oxygen, and 5% carbon dioxide. Embryo evaluation and selection for transfer was carried out on day 3 after oocyte retrieval, where selection was based on developmental stage and morphology. Embryos were ranked according to the number of blastomeres, fragmentation, size equality, and signs of early compaction. Top-ranked embryos consisted of 8 equally sized blastomeres with no to little fragmentation. Supernumerary embryos were cultured until day 4, when selection for cryopreservation was performed based on the degree of embryo compaction and the presence of fragmentation.
Time-Lapse Imaging and Analysis of Morphokinetic Parameters
Embryo images were automatically recorded in seven focal planes (15-μm intervals, 1280 × 1024 pixels, 3 pixels per micrometer, monochrome CCD camera, single red LED 635-nm duration < 0.1 s per image, total light exposure time < 50 s per day per embryo) every 10 min until embryo day 3. Manual annotations were performed by specifically trained members of our team according to the definitions and guidelines by Ciray and colleagues [25]. Time of pronuclei appearance (tPNa) was defined as the appearance of both pronuclei, whereas the time of pronuclei fading (tPNf) was the first frame where both pronuclei were faded. The time points t2, t3, t4, t5, t6, t7 and t8 render the exact timing of reaching the 2, 3, 4, 5, 6, 7- and 8-cell stage of an individual embryo (Fig. 1). Team members performed a proficiency test to check consistency of annotations within and between observers. Extremely close agreement (ICC > 0.95) was observed for the pronuclear stage and the first cleavage divisions until the 5-cell stage, moderate agreement was observed for identifying the 6 to 8cell stage (ICC 0.23–0.40).
Application of the KIDScore Algorithm
To assess embryo quality and the implantation potential of a pre-implantation embryo, the KIDScore was used [12]. The KIDScore ranges from 1 till 5 and is based on six annotations: the number of pronuclei equals 2 at the 1-cell stage, time from insemination to pronuclei fading (tPNf). Time to the 2-, 3-, or 5-cell stage (t2, t3, t5) and the number of cells 66 h after insemination (Fig. 1). The KIDScore is a deselection algorithm, where a decision tree with specific cut-off values determines which score is allocated to an individual embryo. Embryos classified as score 1 show a developmental pattern indicative of a low developmental and implantation potential (an observed average chance of implantation of 5%), whereas score 5 embryos follow a pattern indicative of a high potential (36%) [12].
mHealth Program Smarter Pregnancy
The mHealth program Smarter Pregnancy (https://www.slimmerzwanger.nl; https://www.smarterpregnancy.co.uk) is a (cost) effective tool to improve nutrition and lifestyle [26]. Couples who wanted to participate in the program were subscribed to the mHealth program at the moment of fertility intake in the outpatient clinic of the Erasmus MC University Medical Center, Rotterdam. The Smarter Pregnancy program offers online coaching for a period of 6 months, focusing on four of the most prevalent inadequate behaviors, i.e., vegetables, fruits, alcohol, and folic acid supplement intake, and smoking as the behavior with the strongest detrimental effects on fertility and pregnancy outcome [17, 27,28,29]. The guidelines of the Netherlands Nutrition Centre were used to set the adequate daily intakes of at least 200 g of vegetables, two pieces of fruit, 400 μg of folic acid supplements, no smoking, and no use of alcohol. A baseline questionnaire was used to determine the presence of these five nutrition and lifestyle behaviors, which each were translated in a risk score. A high-risk score represents unhealthy nutrition or lifestyle. Intake of fruits, vegetables, folic acid supplements, and alcohol use was depicted on a scale from 0 to 3 and smoking was depicted on a scale from 0 to 6. Vegetable and fruit intake were both subdivided into a risk score of 0, 1.5, or 3, in which 0 represents an adequate daily intake (Table 1). The total risk score (TRS) was defined as the sum of all risks per behavior. The dietary risk score (DRS) is the combined total of fruits, vegetables, and folic acid supplement intake risk scores, while the lifestyle risk score (LRS) is the combined total of alcohol and smoking risk scores.
Study Parameters
Exposure variables as described above were extracted from the Smarter Pregnancy database. Electronic patient files were used to extract data on age and standardized anthropometric measurements carried out at intake, including maternal height with 0.1-cm accuracy and weight with 0.1-kg accuracy (anthropometric rod and weighing scale; SECA, Hamburg, Germany), as well as information about diagnosis of subfertility and oocyte retrieval date.
As outcome variable, we used the KIDScore as described above. In our hospital, the KIDScore is not used as a decision tool to select embryos for either transfer or cryopreservation. This decision is made by the embryologist based on a single morphological assessment at 66–68 h post fertilization. Only embryos with normal fertilization, as evidenced by the appearance of two pronuclei, that were subsequently transferred or cryopreserved were respectively annotated for research purposes.
Statistical Analysis
Baseline characteristics of the female and male population in the current study are depicted as median or number with the corresponding interquartile range (IQR) or percentage. All analyses were performed using SPSS package 21.0 (IBM SPSS Statistics, Armonk, NY) and R (R: A language and Environment for Statistical Computing, version 3.1.3, 2015 for Windows, R Core Team, Vienna, Austria). To study the association between the nutritional and lifestyle risk scores and the KIDScore in men and women, we used a proportional odds model [30]. This is a model for ordinal outcomes like the KIDScore, using the ordinal package in R (Rune Haubo B Christensen). Challenging in pre-implantation analysis is the fact that couples usually have multiple embryos per cycle and normal regression analysis does not account for this clustering. Random subjects effects are used in the proportional odds model to account for this clustering. To adjust for potential confounders, two different models were constructed for the analysis. In the first model, no adjustments were made (crude model). The second model was adjusted for the covariate maternal age in the study population of women and for maternal age and for the risk score of the corresponding couples’ female risk score in the population of males. Subgroup analyses were performed in women with overweight/obesity (BMI ≥ 25 kg/m2) and normal weight (BMI < 25 kg/m2). The effect estimates of the models were transformed into odds ratios using the exponential function on the effect estimate. This odds ratio represents the chance of an individual embryo proceeding to a 1 point higher KIDScore given the associated maternal risk score. In the proportional odds model this odds ratio is assumed to be constant across all levels of the KIDScore.
To study the association between the fraction of discarded embryos and the nutrition and lifestyle risk scores, we used a generalized linear mixed model (GLMM) approach. Similar to the models discussed above, in the first model no adjustments were made (crude model) where the second model was additionally adjusted for the covariate maternal age. The effect estimates of the models were again transformed into odds ratios using the exponential function on the effect estimate. This odds ratio represents the chance of ICSI treatment resulting in a discarded embryo based on the individual risk score. Since nearly all women used folic acid supplements and nearly none were smokers analyzing these two behaviors was not possible.
Results
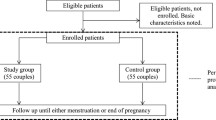
Between January 2014 and December 2017, embryos of 544 couples who underwent their first IVF/ICSI cycle were cultured in the EmbryoScope™. Of these couples, 417 (76.5%) were not subscribed to Smarter Pregnancy. In the end, 113 couples were included, of which 41 of the men also participated. In all included couples, fertilization was performed by using intracytoplasmic sperm injection (ICSI), which resulted in a total of 490 embryos that were cultured in the EmbryoScope™. Of these 490 embryos, 104 embryos were transferred, 254 were frozen, and 132 were of poor quality and thus discarded (Fig. 2). From the 41 couples of which the male partner also participated, a total of 185 embryos were cultured in the EmbryoScope™. Of these 185 embryos (which is a subset of the total amount of 490 embryos), 39 embryos were transferred, 100 were frozen, and 46 were of poor quality and thus discarded (Fig. 2).
Women
The median age and BMI of the women was respectively 32.4 (IQR 29.1–35.1) years and 23.2 (IQR 21.7–26.0) kg/m2, of which 66 (59%) women had a normal BMI and 46 (41%) were overweight/obese (BMI >25 kg/m2). Most women were of Western origin (84%) and were highly educated (51%). The main reason for ICSI treatment was male factor subfertility (81%), of which in 55% of the cases sperm was retrieved surgically. Other indications included unexplained subfertility (2%), female factor subfertility (2%), and combined male-female factor subfertility (15%). The average time between completing the screening of the Smarter Pregnancy program and oocyte retrieval was 49 days (IQR 35–126 days). Most women had inadequate intake of vegetables (n = 79 (72%)) and fruits (n = 63 (58%)). Only two women did not take folic acid supplements (2%). The vast majority of all women did not smoke (n = 107 (98%)) and did not consume alcohol (n = 67 (61%)) (Table 1).
The results from the proportional odds model, indicative of the odds for an individual embryo getting a 1 point higher KIDScore given the individual risk scores, show that the vegetable risk score for the total population of women was negatively associated with the KIDScore with an effect estimate of − 0.28 and an odds ratio of 0.76 (95% CI 0.59 to 0.96) (Table 2a). After adjustment for maternal age, the effect remained statistically significant with an effect estimate of − 0.28 and an odds ratio of 0.76 (95% CI 0.59 to 0.96). No significant associations were observed for fruit intake and alcohol consumption. Furthermore, the DRS was also significantly associated with the KIDScore with an effect estimate of − 0.15 and a corresponding odds ratio of 0.86 (95% CI 0.76 to 0.98). Subgroup analysis showed that the effect of vegetable intake alone was only pronounced in overweight/obese women, with an effect estimate of − 0.55 and an odds ratio of 0.58 (95% CI 0.37 to 0.91). This effect even increased after adjustment for maternal age with an effect estimate of − 0.63 and an odds ratio of 0.53 (95% CI 0.32 to 0.87) (Table 2b). The associations between vegetable intake, fruit intake, and DRS and KIDScore are visually depicted in Supplemental figure 1. In women with a normal BMI, no significant associations were observed between the KIDScore and vegetables and fruit intake and alcohol consumption (Supplemental table 1). When analyzing the risk of developing embryos with poor quality given the individual risk scores, the effect estimate for the TRS was 0.10 with a corresponding odds ratio of 1.11 (95% CI 0.92 to 1.33) after adjustment for maternal age (Supplemental table 2), indicating that the TRS was not associated with the chance of a discarded embryo.
Males
The median age and BMI of the male partner was respectively 34.0 (IQR 29.5–41.5) years and 24.0 (IQR 22.6–27.5). In males, 24 (59%) had a normal BMI (> 18.5 to < 25 kg/m2) and 17 (41%) were overweight. All men were of Western origin and most were highly educated (59%). The main indication for ICSI treatment was male factor subfertility (78%), of which in 53% of the cases sperm was retrieved surgically. The average time between completing the Smarter Pregnancy screening and oocyte retrieval was 45 days (IQR 34.5–97). The vast majority of men had an inadequate intake of vegetables (n = 27 (66%)) and fruits (n = 23 (56%)). A total of 38 (93%) were non-smokers, while refraining from alcohol was reported by only 13 men (32%) (Table 1).
The results from the proportional odds model show that the risk score for smoking was negatively associated with the KIDScore with an effect estimate of − 0.63 and an odds ratio of 0.54 (95% CI 0.34 to 0.85) for the crude model and an effect estimate of − 0.63 and an odds ratio of 0.53 (95% CI 0.33 to 0.85) in the adjusted model, respectively (Table 2c). Although paternal vegetable intake and the DRS and TRS show negative associations with the KIDScore, the effect diminished when adjusting for maternal age combined with the risk scores of women and failed to reach significance.
Discussion
In this study, we showed that inadequate maternal vegetable and fruit intake, as well as paternal smoking during the periconception period, are associated with the quality and developmental morphokinetics of pre-implantation embryos as outcome of implantation potential. Inadequate periconceptional maternal vegetable intake was negatively associated with the quality of resulting ICSI embryos. Moreover, the effect size was more than doubled in women with a BMI > 25 kg/m2. In men, we observed that smoking was negatively associated with embryo quality as measured by the KIDScore. Importantly, our results highlight that a majority of subfertile couples undergoing ICSI treatment are not adherent to a healthy diet and lifestyle in the months preceding oocyte retrieval, semen collection, and subsequent fertilization. Despite the fact that many couples score low on a healthy diet and lifestyle, we did not observe associations between these factors and the proportion of underdevelopment of embryos, which were discarded.
Our results are in line with the study of Braga et al., which showed that periconceptional intake of fruits and vegetables significantly improved pre-implantation embryo quality after ICSI treatment, whereas fruit intake was also positively associated with blastocyst formation [31]. No effects of fruit and vegetable intake were seen regarding clinical ongoing pregnancy rates, which might be attributed to the fact that this study is underpowered for this outcome. However, Braga et al. did not use the KIDScore as outcome parameter, but only performed conventional, static, embryo morphology assessment on day 3. They showed an effect of maternal smoking and alcohol use, although the proportion of women that smoked in their study was not mentioned. The absence of a clear effect of alcohol consumption on pre-implantation embryo quality, could be explained by the fact that in our cohort only a few women reported using alcohol. Moreover, as few women reported smoking and nearly all women used folic acid in our study population, statistical analysis on smoking and folic acid use was not meaningful.
The correlation between a maternal healthy diet and periconceptional outcomes has been well established [3]. Vujkovic et al. showed that adherence to a healthy “Mediterranean” dietary pattern, which consists of high intake of vegetable oils, vegetables, fish, and legumes and low intake of snacks, is associated with an increased chance (odds ratio: 1.4) of pregnancy in couples undergoing an IVF/ICSI treatment [32]. Inversely, an unhealthy diet characterized by low levels of folate, zinc, and antioxidants is associated with a decreased chance of pregnancy [33]. Importantly, a Western diet with high intakes of pizza and potatoes and low intake of fruit was associated with a nearly two-fold increase in developing a congenital cleft lip [34]. In line with these findings, Oostingh et al. recently reported in a review that inadequate maternal nutrition is associated with lower fecundity and that an optimal maternal vitamin status is associated with decreased risk of first-trimester miscarriage [3]. The positive associations in our study between maternal vegetables, fruits, paternal smoking, and pre-implantation embryo quality indicate that both maternal and paternal factors already influence initiation of embryo potential and development capacity directly postconceptionally. These effects could be explained by the fact that fruits and vegetables are rich in exogenous antioxidants, such as vitamin C and vitamin E, and also in elements with antioxidant properties such as folate and zinc. Antioxidants can provide protection of DNA against oxidative stress caused by reactive oxygen species (ROS), which are produced as a byproduct in the process of aerobic metabolism necessary for normal physiological function of DNA replication. However, excessive oxidative stress can result in DNA damage (single and double strand breaks and chromosomal rearrangements) and in sperm also leads to decreased mitochondrial function necessary for seminal propulsion with resulting impaired motility [35]. Interestingly, our results are only present in the overweight/obese group as compared to the normal weight group. Overweight and obesity can be considered a chronic inflammatory state, with fat cells releasing inflammatory factors and thereby inducing a pro-inflammatory state and oxidative stress [36]. Possibly obesity and inadequate fruit and vegetable intake work synergistically and the combination of both induces too much oxidative stress during oogenesis, which results in embryos with less optimal developmental potential.
Paternal smoking and high BMI can have a detrimental effect on all semen parameters like volume, density, concentration and morphology [37, 38], which are linked to reproductive success. It is known that smoking can increase DNA damage and aneuploidies present in sperm and is associated with, or even can be the cause of, congenital malformations [39]. Recent literature shows that DNA damage and sperm epigenetic information are also transferred to the embryo [40, 41]. Our results point to an effect of paternal smoking on sperm that is directly carried over to the pre-implantation embryo as it is associated with less optimal early development and hence lower embryo quality based on the scored morphokinetic parameters. Although we find associations regarding paternal vegetable intake and the embryo KIDScore with similar effect estimates and odds ratios as for the women, these associations disappear when corrected for maternal total risk scores. Since similar odds ratios were seen for men and women, this could possibly be attributed to a power problem. We decided to correct for the maternal risk scores, since we assume that the corresponding effect estimates are due to high correlation between couples regarding their eating habits and lifestyle factors. We are therefore unable to determine who of both does contribute most to the detrimental effects on early developments, the woman or the man. However, since the number of participating women in the current study is far larger than men, we decided to adjust for maternal risk scores in the male analysis. In larger cohorts, we want to argue for the interpretation of the paternal results without corrections for their female partners.
A strength of this study is the use of the validated mHealth Smarter pregnancy program using the dietary risk score. Another strength is the use of the KIDScore to evaluate embryo quality, which is generally used and easily applicable. A KIDScore of 5 is associated with high implantation rates, where a low KIDScore of 1 is associated with low implantation rates. Despite its general and easy applicability, the predictive capability of the KIDScore on implantation has an AUC of 0.65, which could be classified as a fair predictor. In addition, we have to consider for inference of our data that developmental kinetics at the cleavage stages are also reflective of genetic integrity and blastocyst quality. Our study was conducted on embryos cultured until day 3 in a time period that culturing until day 3 was routine clinical practice in most hospitals and also in our hospital. From 2019 onwards, we are culturing the embryos until the blastocyst stage (day 5). Therefore, future research should also investigate the associations between periconceptional parental nutrition and lifestyle factors and the outcome after culturing until day 5, i.e., blastocyst quality, pregnancy rates, and outcomes. In our study we did not investigate the association between nutritional and lifestyle scores on clinical pregnancy rate (detectable embryonic heartbeat), which should be done in larger cohorts. Another strength is that using pre-implantation embryo development in vitro allows studying early development outside of the maternal body, allowing contribution and identification of paternal as well as maternal factors from both parental gametes. The uterine environment makes identification of paternal factors impossible, since factors of the uterine environment could bypass and dampen paternal effects, while following early development and implantation in utero is still technically impossible. Importantly, we show that paternal lifestyle significantly impacts on embryo developmental kinetics during the cleavage stages. Since the study population consists of subfertile couples visiting a tertiary university-based hospital, although it does not mean all couples are in need of tertiary referral or care, the results cannot be automatically extrapolated to the general fertile population and this might have consequences for the external validity of our study. All couples underwent ICSI treatment, so little information is available on IVF and female factor subfertility. However, studying the effects of pre-implantation embryo development can only be performed in fertility centres which have availability of time-lapse imaging. Bias to our results cannot be excluded, because only 36% of the male partners were willing to participate. This study revealed the magnitude of effect sizes which will help us to better calculate the sample size for future larger studies or randomized controlled trials.
We realized that specific subfertility-related diseases, such as endometriosis and polycystic ovary syndrome (PCOS), are also associated with oocyte and embryo quality. Endometriosis negatively affects the oocyte quality, which could be caused by increased oxidative stress [42]. PCOS is associated with decreased embryo quality, defined as slower development during the cleavage stage [43]. Although our study population consisted of 113 women, it was statistically not possible to correct or stratify for these possible confounders. Future studies in larger populations should take these confounders into consideration.
This study showed that both periconceptional maternal and paternal lifestyle and nutritional factors already have an impact on pre-implantation embryo quality based on morphokinetic evaluation after time-lapse culture. As more than 80% of the reproductive population has one or more inadequate nutrition and lifestyle behaviors, studying and modifying the associations by evidence-based interventions is becoming increasingly important. Moreover, the live birth rate per started IVF/ICSI cycle has remained stable at around 30% per cycle for the last decade. With the increase of subfertility in the Western world, it is essential to determine how to stop the increasing subfertility numbers, improve ART chances and prevent and overcome subfertility causes. Therefore, we conclude that identifying modifiable risk factors, as a first step in the behavioral change pathway to stimulate awareness, can have already a clinical impact by increasing the chance of a healthy pregnancy and having a live-born baby.
References
Levine H, Jorgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, et al. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update. 2017;23(6):646–59. https://doi.org/10.1093/humupd/dmx022.
Pal L, Santoro N. Age-related decline in fertility. Endocrinol Metab Clin N Am. 2003;32(3):669–88.
Oostingh EC, Hall J, Koster MPH, Grace B, Jauniaux E, Steegers-Theunissen RPM. The impact of maternal lifestyle factors on periconception outcomes: a systematic review of observational studies. Reprod BioMed Online. 2019;38(1):77–94. https://doi.org/10.1016/j.rbmo.2018.09.015.
Wong KM, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to in vitro fertilization success rates. Fertil Steril. 2014;102(1):19–26. https://doi.org/10.1016/j.fertnstert.2014.05.027.
Freour T, Lammers J, Splingart C, Jean M, Barriere P. Time lapse (Embryoscope(R)) as a routine technique in the IVF laboratory: a useful tool for better embryo selection?] L'observation en continu du developpement embryonnaire en FIV (time lapse) a l'aide de l'Embryoscope((R)) : un outil d'aide a la decision? Gynecol Obstet Fertil. 2012;40(9):476–80. https://doi.org/10.1016/j.gyobfe.2012.07.008.
Goodman LR, Goldberg J, Falcone T, Austin C, Desai N. Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertil Steril. 2016;105(2):275–85 e10. https://doi.org/10.1016/j.fertnstert.2015.10.013.
Neuber E, Rinaudo P, Trimarchi JR, Sakkas D. Sequential assessment of individually cultured human embryos as an indicator of subsequent good quality blastocyst development. Hum Reprod. 2003;18(6):1307–12.
Wolff HS, Fredrickson JR, Walker DL, Morbeck DE. Advances in quality control: mouse embryo morphokinetics are sensitive markers of in vitro stress. Hum Reprod. 2013;28(7):1776–82. https://doi.org/10.1093/humrep/det102.
D'Souza F, Pudakalakatti SM, Uppangala S, Honguntikar S, Salian SR, Kalthur G, et al. Unraveling the association between genetic integrity and metabolic activity in pre-implantation stage embryos. Sci Rep. 2016;6:37291. https://doi.org/10.1038/srep37291.
Lee YS, Thouas GA, Gardner DK. Developmental kinetics of cleavage stage mouse embryos are related to their subsequent carbohydrate and amino acid utilization at the blastocyst stage. Hum Reprod. 2015;30(3):543–52. https://doi.org/10.1093/humrep/deu334.
Weinerman R, Feng R, Ord TS, Schultz RM, Bartolomei MS, Coutifaris C, et al. Morphokinetic evaluation of embryo development in a mouse model: functional and molecular correlates. Biol Reprod. 2016;94(4):84. https://doi.org/10.1095/biolreprod.115.134080.
Petersen BM, Boel M, Montag M, Gardner DK. Development of a generally applicable morphokinetic algorithm capable of predicting the implantation potential of embryos transferred on day 3. Hum Reprod. 2016;31(10):2231–44. https://doi.org/10.1093/humrep/dew188.
Adolfsson E, Porath S, Andershed AN. External validation of a time-lapse model; a retrospective study comparing embryo evaluation using a morphokinetic model to standard morphology with live birth as endpoint. JBRA Assist Reprod. 2018;22(3):205–14. https://doi.org/10.5935/1518-0557.20180041.
Mook-Kanamori DO, Steegers EA, Eilers PH, Raat H, Hofman A, Jaddoe VW. Risk factors and outcomes associated with first-trimester fetal growth restriction. JAMA. 2010;303(6):527–34. https://doi.org/10.1001/jama.2010.78.
Steegers-Theunissen RP, Twigt J, Pestinger V, Sinclair KD. The periconceptional period, reproduction and long-term health of offspring: the importance of one-carbon metabolism. Hum Reprod Update. 2013;19(6):640–55. https://doi.org/10.1093/humupd/dmt041.
Temel S, van Voorst SF, Jack BW, Denktas S, Steegers EA. Evidence-based preconceptional lifestyle interventions. Epidemiol Rev. 2014;36:19–30. https://doi.org/10.1093/epirev/mxt003.
van Uitert EM, van der Elst-Otte N, Wilbers JJ, Exalto N, Willemsen SP, Eilers PH, et al. Periconception maternal characteristics and embryonic growth trajectories: the Rotterdam Predict study. Hum Reprod. 2013;28(12):3188–96. https://doi.org/10.1093/humrep/det375.
Hammiche F, Laven JS, van Mil N, de Cock M, de Vries JH, Lindemans J, et al. Tailored preconceptional dietary and lifestyle counselling in a tertiary outpatient clinic in The Netherlands. Hum Reprod. 2011;26(9):2432–41. https://doi.org/10.1093/humrep/der225.
Twigt JM, Bolhuis ME, Steegers EA, Hammiche F, van Inzen WG, Laven JS, et al. The preconception diet is associated with the chance of ongoing pregnancy in women undergoing IVF/ICSI treatment. Hum Reprod. 2012;27(8):2526–31. https://doi.org/10.1093/humrep/des157.
Chan KA, Tsoulis MW, Sloboda DM. Early-life nutritional effects on the female reproductive system. J Endocrinol. 2015;224(2):R45–62. https://doi.org/10.1530/JOE-14-0469.
Hoek J, Koster MPH, Schoenmakers S, Willemsen SP, Koning AHJ, Steegers EAP, et al. Does the father matter? The association between the periconceptional paternal folate status and embryonic growth. Fertil Steril. 2019;111(2):270–9. https://doi.org/10.1016/j.fertnstert.2018.10.017.
Vujkovic M, de Vries JH, Dohle GR, Bonsel GJ, Lindemans J, Macklon NS, et al. Associations between dietary patterns and semen quality in men undergoing IVF/ICSI treatment. Hum Reprod. 2009;24(6):1304–12. https://doi.org/10.1093/humrep/dep024.
van Dijk MR, Oostingh EC, Koster MP, Willemsen SP, Laven JS, Steegers-Theunissen RP. The use of the mHealth program Smarter Pregnancy in preconception care: rationale, study design and data collection of a randomized controlled trial. BMC Pregnancy Childbirth. 2017;17(1):46. https://doi.org/10.1186/s12884-017-1228-5.
Hohmann FP, Macklon NS, Fauser BC. A randomized comparison of two ovarian stimulation protocols with gonadotropin-releasing hormone (GnRH) antagonist cotreatment for in vitro fertilization commencing recombinant follicle-stimulating hormone on cycle day 2 or 5 with the standard long GnRH agonist protocol. J Clin Endocrinol Metab. 2003;88(1):166–73. https://doi.org/10.1210/jc.2002-020788.
Ciray HN, Campbell A, Agerholm IE, Aguilar J, Chamayou S, Esbert M, et al. Proposed guidelines on the nomenclature and annotation of dynamic human embryo monitoring by a time-lapse user group. Hum Reprod. 2014;29(12):2650–60. https://doi.org/10.1093/humrep/deu278.
Oostingh EC, Ophuis RH, Koster MP, Polinder S, Lingsma HF, Laven JS, et al. Mobile health coaching on nutrition and lifestyle behaviors for subfertile couples using the smarter pregnancy program: model-based cost-effectiveness analysis. JMIR Mhealth Uhealth. 2019;7(10):e13935. https://doi.org/10.2196/13935.
Chaudhuri JD. Alcohol and the developing fetus--a review. Med Sci Monit. 2000;6(5):1031–41.
Englund-Ogge L, Brantsaeter AL, Sengpiel V, Haugen M, Birgisdottir BE, Myhre R, et al. Maternal dietary patterns and preterm delivery: results from large prospective cohort study. BMJ. 2014;348:g1446. https://doi.org/10.1136/bmj.g1446.
Jaddoe VW, Verburg BO, de Ridder MA, Hofman A, Mackenbach JP, Moll HA, et al. Maternal smoking and fetal growth characteristics in different periods of pregnancy: the generation R study. Am J Epidemiol. 2007;165(10):1207–15. https://doi.org/10.1093/aje/kwm014.
McCullagh P. Regression models for ordinal data. J R Stat Soc. 1980;42(2):109–42.
Braga DP, Halpern G, Setti AS, Figueira RC, Iaconelli A Jr, Borges E Jr. The impact of food intake and social habits on embryo quality and the likelihood of blastocyst formation. Reprod BioMed Online. 2015;31(1):30–8. https://doi.org/10.1016/j.rbmo.2015.03.007.
Vujkovic M, de Vries JH, Lindemans J, Macklon NS, van der Spek PJ, Steegers EA, et al. The preconception Mediterranean dietary pattern in couples undergoing in vitro fertilization/intracytoplasmic sperm injection treatment increases the chance of pregnancy. Fertil Steril. 2010;94(6):2096–101. https://doi.org/10.1016/j.fertnstert.2009.12.079.
Ebisch IM, Thomas CM, Peters WH, Braat DD, Steegers-Theunissen RP. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum Reprod Update. 2007;13(2):163–74. https://doi.org/10.1093/humupd/dml054.
Vujkovic M, Ocke MC, van der Spek PJ, Yazdanpanah N, Steegers EA, Steegers-Theunissen RP. Maternal Western dietary patterns and the risk of developing a cleft lip with or without a cleft palate. Obstet Gynecol. 2007;110(2 Pt 1):378–84. https://doi.org/10.1097/01.AOG.0000268799.37044.c3.
Wagner H, Cheng JW, Ko EY. Role of reactive oxygen species in male infertility: an updated review of literature. Arab J Urol. 2018;16(1):35–43. https://doi.org/10.1016/j.aju.2017.11.001.
Ellulu MS, Patimah I, Khaza'ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13(4):851–63. https://doi.org/10.5114/aoms.2016.58928.
Du Plessis SS, Cabler S, McAlister DA, Sabanegh E, Agarwal A. The effect of obesity on sperm disorders and male infertility. Nat Rev Urol. 2010;7(3):153–61. https://doi.org/10.1038/nrurol.2010.6.
Li Y, Lin H, Li Y, Cao J. Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil Steril. 2011;95(1):116–23. https://doi.org/10.1016/j.fertnstert.2010.06.031.
Beal MA, Yauk CL, Marchetti F. From sperm to offspring: assessing the heritable genetic consequences of paternal smoking and potential public health impacts. Mutat Res. 2017;773:26–50. https://doi.org/10.1016/j.mrrev.2017.04.001.
Carrell DT. The sperm epigenome: implications for assisted reproductive technologies. Adv Exp Med Biol. 2019;1166:47–56. https://doi.org/10.1007/978-3-030-21664-1_3.
Casanovas A, Ribas-Maynou J, Lara-Cerrillo S, Jimenez-Macedo AR, Hortal O, Benet J, et al. Double-stranded sperm DNA damage is a cause of delay in embryo development and can impair implantation rates. Fertil Steril. 2019;111(4):699–707 e1. https://doi.org/10.1016/j.fertnstert.2018.11.035.
Da Broi MG, Navarro PA. Oxidative stress and oocyte quality: ethiopathogenic mechanisms of minimal/mild endometriosis-related infertility. Cell Tissue Res. 2016;364(1):1–7. https://doi.org/10.1007/s00441-015-2339-9.
Wissing ML, Bjerge MR, Olesen AI, Hoest T, Mikkelsen AL. Impact of PCOS on early embryo cleavage kinetics. Reprod BioMed Online. 2014;28(4):508–14. https://doi.org/10.1016/j.rbmo.2013.11.017.
Acknowledgments
The authors thank Eline Oostingh, MD, and Matthijs van Dijk, MD, PhD, for their contribution to the data acquisition and Sanne Overdijkink, MD, and Linda van Opstal, MSc, for their contribution to the conceptualization of this study.
Availability of Data and Material
Data are available upon request.
Funding
This research was funded by the Department of Obstetrics and Gynecology of the Erasmus MC, University Medical Center, Rotterdam, the Netherlands; The Netherlands Organization for Health Research and Development (ZonMW, project number 209040003); and the Erasmus MC Medical Research Advisor Committee’s “Health Care Efficiency Research” program.
Author information
Authors and Affiliations
Contributions
EB was responsible for timelapse imaging. JL was responsible for IVF patients. RST initiated the research question and supervised all aspects of the study. JH, EB, and EvM contributed to data acquisition. SW and RST initiated and supervised the statistical procedures of the manuscript. JH, SS, and MK wrote the first draft. All authors contributed to the writing and the critical revisions of the manuscript and all authors approved the final version of the manuscript and authorized the submitted version.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving patients were approved by the Medical Ethical Institutional Review Board of the Erasmus, University Medical Center, Rotterdam, the Netherlands.
Consent to Participate
Written informed consent was obtained from all female and male participants at enrolment.
Consent for Publication
Not applicable.
Code Availability
Data are available upon request.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hoek, J., Schoenmakers, S., Baart, E.B. et al. Preconceptional Maternal Vegetable Intake and Paternal Smoking Are Associated with Pre-implantation Embryo Quality. Reprod. Sci. 27, 2018–2028 (2020). https://doi.org/10.1007/s43032-020-00220-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00220-8