Abstract
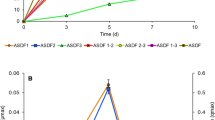
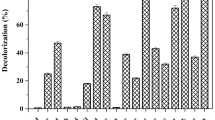
Biodegradation of reactive azo dyes has been an arduous problem for decades. Several efficient biosystems have been proposed for dye degradation, but most of them are dependent on the availability of costly co-substrates such as peptone, yeast extract, and/or glucose. The present study describes the azo dye degradation by a bacterial consortium using glycerol as a sole co-substrate. The consortium was developed from a mixed bacterial culture obtained upon enrichment of soil sediment for Reactive Blue 28 (RB28) decolorization in the presence of glycerol (0.1%; v/v). The consortium with three bacterial species, i.e., Stenotrophomonas acidaminiphila APG1, Cellulomonas sp. APG4, and Pseudomonas stutzeri APG2, designated as “SCP,” decolorized 92% of 100 ppm dye in 96 h. The intricacies of the interactions existing within the members of the consortium were resolved by a simple and unique analysis called “BSocial.” Among all the members, Cellulomonas sp. APG4 exerted a net-positive impact for decolorization (%) on the consortium. The net fitness of the community increased when all the three species were present, and thus, all of them were selected for further analysis. Moreover, APG4 seemed to be central in the reductive decolorization as it possessed the highest reductase activity. The dye degradation by the consortium was demonstrated by UV-Visible spectroscopy, HPTLC, and FTIR spectroscopy of control and decolorized cell-free supernatant. The LC-ESI-MS analysis of metabolites extracted from decolorized cell-free medium led to the identification of degradation products, thus leading us to propose the plausible pathway for degradation of RB28 by bacterial consortium.








Similar content being viewed by others
References
Sharma SCD, Sun Q, Li J, Wang Y, Suanon F, Yang J, Yu CP (2016) Decolorization of azo dye methyl red by suspended and co-immobilized bacterial cells with mediators anthraquinone-2,6-disulfonate and Fe3O4 nanoparticles. Int Biodeterior Biodegrad 112:88–97. https://doi.org/10.1016/j.ibiod.2016.04.035
Krishnamoorthy R, Jose PA, Ranjith M, Anandham R, Suganya K, Prabhakaran J, Thiyageshwari S, Johnson J, Gopal NO, Kumutha K (2018) Decolourisation and degradation of azo dyes by mixed fungal culture consisted of Dichotomomyces cejpii MRCH 1-2 and Phoma tropica MRCH 1-3. J Environ Chem Eng 6(1):588–595. https://doi.org/10.1016/j.jece.2017.12.035
Maas R, Chaudhari S (2005) Adsorption and biological decolourization of azo dye reactive red 2 in semicontinuous anaerobic reactors. Process Biochem 40(2):699–705. https://doi.org/10.1016/j.procbio.2004.01.038
Saratale RG, Saratale GD, Chang JS, Govindwar SP (2011) Bacterial decolorization and degradation of azo dyes : a review. J Taiwan Inst Chem Eng 42(1):138–157
Dafale N, Rao NN, Meshram SU, Wate SR (2008) Decolorization of azo dyes and simulated dye bath wastewater using acclimatized microbial consortium – biostimulation and halo tolerance. Bioresour Technol 99:2552–2558. https://doi.org/10.1016/j.biortech.2007.04.044
Keharia H, Madamwar D (2003) Bioremediation concepts for treatment of dye containing wastewater : a review. Indian J Exp Biol 4:1068–1075
Holkar CR, Jadhav AJ, Pinjari DV, Mahamuni NM, Pandit AB (2016) A critical review on textile wastewater treatments: possible approaches. J Environ Manag 182:351–366. https://doi.org/10.1016/j.jenvman.2016.07.090
Watanabe K, Manefield M, Lee M, Kouzuma A (2009) Electron shuttles in biotechnology. Curr Opin Biotechnol 20(6):633–641. https://doi.org/10.1016/j.copbio.2009.09.006
Hernandez ME, Newman DK (2001) Extracellular Electron Transfer. Cell Mol Life Sci 50(2):1562–1571. https://doi.org/10.1128/AEM.70.2.921
Stolz A (2001) Basic and applied aspects in the microbial degradation of azo dyes. Appl Microbiol Biotechnol 56(1–2):69–80. https://doi.org/10.1007/s002530100686
Pandey A, Singh P, Iyengar L (2007) Bacterial decolorization and degradation of azo dyes. Int Biodeterior Biodegrad 59:73–84. https://doi.org/10.1016/j.ibiod.2006.08.006
Brenner K, You L, Arnold FH (2008) Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol 26(9):483489–483489. https://doi.org/10.1016/j.tibtech.2008.05.004
Shong J, Diaz MRF, Collins CH (2012) Towards synthetic microbial consortia for bioprocessing. Curr Opin Biotechnol 23(5):798–802. https://doi.org/10.1016/j.copbio.2012.02.001
Senan RC, Abraham TE (2004) Bioremediation of textile azo dyes by aerobic bacterial consortium. Biodegrad 15:275–280. https://doi.org/10.1023/B:BIOD.0000043000.18427.0a
Xu H, Yang B, Liu Y, Li F, Shen C, Ma C, Tian Q, Song X, Sand W (2018) Recent advances in anaerobic biological processes for textile printing and dyeing wastewater treatment: a mini-review. World J Microbiol Biotechnol 34(11):165. https://doi.org/10.1007/s11274-018-2548-y
Van der Zee FP, Villaverde S (2005) Combined anaerobic–aerobic treatment of azo dyes—a short review of bioreactor studies. Water Res 39(8):1425–1440
Yan LK, Fung KY, Ng KM (2018) Aerobic sludge granulation for simultaneous anaerobic decolorization and aerobic aromatic amines mineralization for azo dye wastewater treatment. Environ Technol 39(11):1368–1375. https://doi.org/10.1080/09593330.2017.1329354
Jayapal M, Jagadeesan H, Shanmugam M, Murugesan S (2018) Sequential anaerobic-aerobic treatment using plant microbe integrated system for degradation of azo dyes and their aromatic amines by-products. J Hazard Mater 354:231–243. https://doi.org/10.1016/j.jhazmat.2018.04.050
Masarbo RS, Ismailsab M, Monisha TR, Nayak AS, Karegoudar TB (2018) Enhanced decolorization of sulfonated azo dye methyl orange by single and mixed bacterial strains AK1, AK2 and VKY1. Bioremediat J 22(3–4):136–146. https://doi.org/10.1080/10889868.2018.1516612
Nigam P, Mc Mullan G, Banat IM, Marchant R (1996) Decolourisation of effluent from the textile industry by a microbial consortium. Biotechnol Lett 18(1):117–120. https://doi.org/10.1007/BF00137823
Balapure KH, Jain K, Chattaraj S, BhattNS MD (2014) Co-metabolic degradation of diazo dye — reactive blue 160 by enriched mixed cultures BDN. J Hazard Mater 279:85–95. https://doi.org/10.1016/j.jhazmat.2014.06.057
Nikhil B, Sapna T, Kshama B (2012) Biodegradation of reactive red M8B by bacterial consortium SpNb1. Indian J Sci Technol 5(7):3047–3053
Cheriaa J, Khaireddine M, Rouabhia M, Bakhrouf A (2012) Removal of triphenylmethane dyes by bacterial consortium. Sci World J 2012:1–9. https://doi.org/10.1100/2012/512454
Ghanem KM, Al-fassi FA, Biag AK (2012) Optimization of methyl orange decolorization by mono and mixed bacterial culture techniques using statistical designs. Afr J Microbiol Res 6(12):2918–2928. https://doi.org/10.5897/AJMR11.1390
Joshi T, Iyengar L, Singh K, Garg S (2008) Isolation, identification and application of novel bacterial consortium TJ-1 for the decolourization of structurally different azo dyes. Bioresour Technol 99:7115–7121. https://doi.org/10.1016/j.biortech.2007.12.074
Khehra MS, Saini HS, Sharma DK, Chadha BS, Chimni SS (2005) Comparative studies on potential of consortium and constituent pure bacterial isolates to decolorize azo dyes. Water Res 39(20):5135–5141. https://doi.org/10.1016/j.watres.2005.09.033
Jian-bo GUO, Ji-ti ZHOU, Dong WANG, Jing WANG, Hui YU, Zhi-yong SONG (2005) Decolorization of azo dyes with high salt concentration by salt-tolerant mixed cultures under anaerobic conditions. J Environ Sci 17(6):984–988
Mishra S, Maiti A (2018) The efficacy of bacterial species to decolourise reactive azo, anthroquinone and triphenylmethane dyes from wastewater: a review. Environ Sci Pollut Res 25(9):8286–8314. https://doi.org/10.1007/s11356-018-1273-2
Kong Z, Li L, Xue Y, Yang M, Li YY (2019) Challenges and prospects for the anaerobic treatment of chemical-industrial organic wastewater: a review. J Clean Prod 231:913–927. https://doi.org/10.1016/j.jclepro.2019.05.233
Paulo G, Mack M, Contiero J (2009) Glycerol : a promising and abundant carbon source for industrial microbiology. Biotechnol Adv 27(1):30–39. https://doi.org/10.1016/j.biotechadv.2008.07.006
Pagliaro M, Ciriminna R, Kimura H, Rossi M, Della Pina C (2007) From glycerol to value-added products. Angew Chem Int Ed 46(24):4434–4440. https://doi.org/10.1002/anie.200604694
Purswani J, Romero-Zaliz RC, Martín-Platero AM, Guisado IM, González-López J, Pozo C (2017) BSocial: deciphering social behaviors within mixed microbial populations. Front Microbiol 8:919. https://doi.org/10.3389/fmicb.2017.00919
RStudio Team (2015) RStudio: Integrated development for R. RStudio, Inc., Boston, MA URL http://www.rstudio.com, 42, 14
Warnes GR, Bolker B, Bonebakker L, Gentleman R, Huber W, Liaw A, Lumley T, Maechler M, Magnusson A, Moeller S (2013). gplots: Various R programming tools for plotting data. R package version 2.12. 1. Available online at: http. CRAN. R-project. org/package= gplots
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729. https://doi.org/10.1093/molbev/mst197
Ayed L, Khelifi E, Jannet HB, Miladi H, Cheref A, Achour S, Bakhrouf A (2010) Response surface methodology for decolorization of azo dye methyl orange by bacterial consortium: produced enzymes and metabolites characterization. Chem Eng J 165(1):200–208. https://doi.org/10.1016/j.cej.2010.09.018
Maqbool Z, Hussain S, Ahmad T, Nadeem H, Imran M, Khalid A, Abid M, Martin-Laurent F (2016) Use of RSM modeling for optimizing decolorization of simulated textile wastewater by Pseudomonas aeruginosa strain ZM130 capable of simultaneous removal of reactive dyes and hexavalent chromium. Environ Sci Pollut Res 23(11):11224–11239. https://doi.org/10.1007/s11356-016-6275-3
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Yazdani SS, Gonzalez R (2007) Anaerobic fermentation of glycerol: a path to economic viability for the biofuels industry. Curr Opin Biotechnol 18(3):213–219. https://doi.org/10.1016/j.copbio.2007.05.002
Pearce CI, Christie R, Boothman C, von Canstein H, Guthrie JT, Lloyd JR (2006) Reactive azo dye reduction by Shewanella strain J18 143. Biotechnol Bioeng 95(4):692–703. https://doi.org/10.1002/bit.21021
Chengalroyen MD, Dabbs ER (2013) The microbial degradation of azo dyes : minireview. World J Microbiol Biotechnol 29:389–399. https://doi.org/10.1007/s11274-012-1198-8
Horemans B, Smolders E, Springael D (2013) Carbon source utilization profiles suggest additional metabolic interactions in a synergistic linuron-degrading bacterial consortium. FEMS Microbiol Eco 84(1):24–34. https://doi.org/10.1111/1574-6941.12033
Özkaya Ö, Xavier KB, Dionisio F, Balbontín R (2017) Maintenance of microbial cooperation mediated by public goods in single- and multiple-trait scenarios. J Bacteriol 199(22):1–14. https://doi.org/10.1128/JB.00297-17
Jadhav JP, Kalyani DC, Telke AA, Phugare SS, Govindwar SP (2010) Evaluation of the efficacy of a bacterial consortium for the removal of color, reduction of heavy metals, and toxicity from textile dye effluent. Bioresour Technol 101(1):165–173. https://doi.org/10.1016/j.biortech.2009.08.027
Saratale RG, Saratale GD, Govindwar SP, Kim DS (2014) Exploiting the efficacy of Lysinibacillus sp. RGS for decolorization and detoxification of industrial dyes, textile effluent and bioreactor studies. J Environ Sci Health A 50(2):172–196. https://doi.org/10.1080/10934529.2014.975536
Waghmode TR, Kurade MB, Kagalkar AN, Govindwar SP (2012) Differential fate of metabolism of a disperse dye by microorganisms Galactomyces geotrichum and Brevibacillus laterosporus and their consortium GG-BL. J Environ Sci 24(7):1295–1304. https://doi.org/10.1016/S1001-0742(11)60899-1
Purswani J, Guisado IM, Coello-Cabezas J, González-López J, Pozo C (2019) Social microbial inocula confer functional stability in a methyl tert-butyl ether extractive membrane biofilm bioreactor. Environ Pollut 244:855–860. https://doi.org/10.1016/j.envpol.2018.10.100
Glasl B, Smith CE, Bourne DG, Webster NS (2018) Exploring the diversity-stability paradigm using sponge microbial communities. Sci Rep 8(1):8425. https://doi.org/10.1038/s41598-018-26641-9
McCann KS (2000 May) The diversity–stability debate. Nature. 405(6783):228–233. https://doi.org/10.1038/35012234
Zahran SA, Ali-Tammam M, Hashem AM, Aziz RK, Ali AE (2019) Azoreductase activity of dye-decolorizing bacteria isolated from the human gut microbiota. Sci Rep 9(1):5508. https://doi.org/10.1038/s41598-019-41894-8
Chauhan PS, Goradia B, Saxena A (2017) Bacterial laccase: recent update on production, properties and industrial applications. 3. Biotech 7(5):323. https://doi.org/10.1007/s13205-017-0955-7
Mahmood S, Khalid A, Arshad M, Mahmood T, Crowley DE (2016) Detoxification of azo dyes by bacterial oxidoreductase enzymes. Crit Rev Biotechnol 36(4):639–651. https://doi.org/10.3109/07388551.2015.1004518
Çifçi Dİ, Atav R, Güneş Y, Güneş E (2019) Determination of the color removal efficiency of laccase enzyme depending on dye class and chromophore. Water Sci Technol 80(1):134–143. https://doi.org/10.2166/wst.2019.255
Bankole PO, Adekunle AA, Govindwar SP (2019) Demethylation and desulfonation of textile industry dye, Thiazole Yellow G by Aspergillus niger LAG. Biotechnol Rep 23:e00327. https://doi.org/10.1016/j.btre.2019.e00327
Van der Zee FP, Cervantes FJ (2009) Impact and application of electron shuttles on the redox (bio) transformation of contaminants : a review. Biotechnol Adv 27:256–277. https://doi.org/10.1016/j.biotechadv.2009.01.004
Phugare SS, Kalyani DC, Patil AV, Jadhav JP (2011) Textile dye degradation by bacterial consortium and subsequent toxicological analysis of dye and dye metabolites using cytotoxicity, genotoxicity and oxidative stress studies. J Hazard Mater 186(1):713–723. https://doi.org/10.1016/j.jhazmat.2010.11.049
Acknowledgments
The authors are also grateful to CISST, Sardar Patel University, Vallabh Vidyanagar, Gujarat, India, for providing analytical facilities. Sandhya Nanjani gratefully acknowledges the University Grants Commission, New Delhi, for the award of SRF to pursue doctoral studies.
Funding
The authors acknowledge the funds received from the Department of Biotechnology-Centre of Excellence and Innovation in Biotechnology (DBT-CEIB) and Department of Science and Technology-Fund for Improvement of S&T Infrastructure in Universities and Higher Educational Institutions (DST-FIST) Program, Ministry of Science and Technology, and CAS program funded by UGC, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Cyntia Canedo Silva
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 769 kb)
Rights and permissions
About this article
Cite this article
Nanjani, S., Rawal, K. & Keharia, H. Decoding social behaviors in a glycerol dependent bacterial consortium during Reactive Blue 28 degradation. Braz J Microbiol 51, 1837–1851 (2020). https://doi.org/10.1007/s42770-020-00303-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-020-00303-3