Abstract
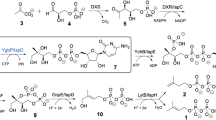
Bacterial resistance towards aminoglycoside antibiotics mainly occurs because of aminoglycoside phosphotransferases (APHs). It is thus necessary to provide a rationale for focusing inhibitor development against APHs. The nucleotide triphosphate (NTP) binding site of eukaryotic protein kinases (ePKs) is structurally conserved with APHs. However, ePK inhibitors cannot be used against APHs due to cross reactivity. Thus, understanding bacterial resistance at the atomic level could be useful to design new inhibitors against such resistant pathogens. Hence, we carried out in vitro studies of APH from newly deposited multidrug-resistant organism Bacillus subtilis subsp. subtilis strain RK. Enzymatic modification studies of different aminoglycoside antibiotics along with purification and characterization revealed a novel class of APH, i.e., APH(5), with molecular weight 27 kDa approximately. Biochemical analysis of virtually screened inhibitor ZINC71575479 by coupled spectrophotometric assay showed complete enzymatic inhibition of purified APH(5). In silico toxicity study comparison of ZINC71575479 with known inhibitor of APH, i.e., tyrphostin AG1478, predicted its acceptable values for 96 h fathead minnow LC50, 48 h Tetrahymena pyriformis IGC50, oral rat LD50, and developmental toxicity using different QSAR methodologies. Thus, the present study gives novel insight into the aminoglycoside resistance and inhibition mechanism of APH(5) by applying experimental and computational techniques synergistically.









Similar content being viewed by others
References
Arias CA, Murray BE (2009) Antibiotic-resistant bugs in the 21st century--a clinical super-challenge. N Engl J Med 360:439–443
Davies J (1994) Inactivation of antibiotics and the dissemination of resistance genes. Science 264:375–382
Bryskier A (2005) Antibiotics and antibacterial agents: classifications and structure activity relationships. Antimicrobial Agents ASM Press,Washington, DC p.13–38
Cox G, Stogios P, Savchenko A, Wright GD (2015) Structural and molecular basis for resistance to aminoglycoside antibiotics by the adenylyltransferase ANT(2″)-Ia. MBio. 6:e02180–e02114. https://doi.org/10.1128/mBio.02180-14
Yao J, Moellering R (2007) Antibacterial agents. Manual of clinical microbiology. American Society for Microbiology Press, Washington, DC p.1077–1113
Alekshun MN, Levy SB (2007) Molecular mechanisms of antibacterial multidrug resistance. Cell 128:1037–1050
Hancock RE (1981) Aminoglycoside uptake and mode of action--with special reference to streptomycin and gentamicin. I Antagonists and mutants. J Antimicrob Chemother 8:249–276
Galimand M, Sabtcheva S, Courvalin P, Lambert T (2005) Worldwide disseminated armA aminoglycoside resistance methylase gene is borne by composite transposon Tn1548. Antimicrob Agents Chemother 49:2949–2953
Aires JR, Kohler T, Nikaido H, Plesiat P (1999) Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob Agents Chemother 43:2624–2628
Ramirez MS, Tolmasky ME (2010) Aminoglycoside modifying enzymes. Drug Resist Updat 13:151–171
Shi K, Berghuis AM (2012) Structural basis for dual nucleotide selectivity of aminoglycoside 2″-phosphotransferase IVa provides insight on determinants of nucleotide specificity of aminoglycoside kinases. J Biol Chem 287:13094–13102
Hon WC, McKay GA, Thompson PR, Sweet RM, Yang DS, Wright GD, Berghuis AM (1997) Structure of an enzyme required for aminoglycoside antibiotic resistance reveals homology to eukaryotic protein kinases. Cell 89:887–895
Liao JJ (2007) Molecular recognition of protein kinase binding pockets for design of potent and selective kinase inhibitors. J Med Chem 50:409–424
Turnbull PC, Kramer J, Melling J (1990) Bacillus, sp In Topley and Wilson’s principles of bacteriology, virology and immunity, vol. 2, 8th ed. Edward Arnold, London. p. 188–210
Farrar WE (1963) Serious infections due to “non-pathogenic” organisms of the genus Bacillus. Review of their status as pathogens. Am J Med 34:134–141
Parulekar RS, Sonawane KD (2017) Molecular modeling studies to explore the binding affinity of virtually screened inhibitor toward different aminoglycoside kinases from diverse MDR strains. J Cell Biochem 119:2679–2695
Ericsson HM, Sherris JC (1971) Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathol Microbiol Scand B Microbiol Immunol 217: (Suppl.) 1S
Peixoto RS, da Costa Coutinho HL, Rumjanek NG, Macrae A, Rosado AS (2002) Use of rpoB and 16S rRNA genes to analyse bacterial diversity of a tropical soil using PCR and DGGE. Lett Appl Microbiol 35:316–320
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins GD (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Clinical and Laboratory Standards Institute (2006) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. 7thed. Document M7-A7.Wayne, PA: CLSI
Klundert JA, Vligenthart JS, van Doorn E, Bongaerts GP, Molendijk L, Mouton RP (1984) A simple method for the identification of aminoglycoside modifying enzymes. J Antimicrob Chemother 14:339–348
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Ubukata K, Yamashita N, Gotoh A, Konno M (1984) Purification and characterization of aminoglycoside mofifying enzymes from Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother 25:754–759
Dhote V, Gupta S, Reynolds KA (2008) An O-phosphotransferase catalyzes phosphorylation of hygromycin A in the antibiotic- producing Streptomyces hygroscopicus. Antimicrob Agents Chemother 52:3580–3588
Ashenafi M, Ammosova T, Nekhai S, Byrnes WB (2014) Purification and characterization of aminoglycoside phosphotransferase APH(6)-Id, a streptomycin-inactivating enzyme. Mol Cell Biochem 387:207–216
Thompson CJ, Skinner RH, Thompson J, Ward JM, Hopwood DA, Cundliffe E (1982) Biochemical characterization of resistance determinanats cloned from antibiotic- producing streptomycetes. J Bacteriol 151:678–685
Parulekar RS, Sonawane KD (2018) Insights into the antibiotic resistance and inhibition mechanism of aminoglycoside phosphotransferase from Bacillus cereus: in silico and in vitro perspective. J Cell Biochem 119:9444–9461
Martin T (2016) U.S EPA. User’s guide for T.E.S.T. (version 4.2) (toxicity estimation software tool): a program to estimate toxicity from molecular structure
Daigle DM, Mckay GA, Wright GD (1997) Inhibition of aminoglycoside antibiotic resistance enzymes by protein kinase inhibitors. J Biol Chem 272:24755–24758
Bottone EJ (2010) Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev 23:382–338
Turnbull PC, Sirianni NM, LeBron CI, Samaan MN, Sutton FN, Reyes AE, Peruski AF Jr (2004) MICs of selected antibiotics for Bacillus anthracis, Bacillus cereus, Bacillus thuringiensis, and Bacillus mycoides from a range of clinical and environmental sources as determined by the Etest. J Clin Microbiol 42:3626–3634
Murray BE, Moellering RC (1979) Aminoglycoside-modifying enzymes among clinical isolates of Acinetobacter calcoaceticus subsp. Anitratus (Herelleavaginicola): explanation for high-level aminoglycoside resistance. Antimicrob Agents Chemother 15:190–199
Zhang LI, Li X, Poole K (2000) Multiple antibiotic resistance in Stenotrophomonas maltophilia involvement of a multidrug efflux system. Antimicrob Agents Chemother 44:287–293
Wright GD, Ladak P (1997) Overexpression and characterization of the chromosomal aminoglycoside 6′- N- acetyltransferase from Enterococcus faecium. Antimicrob Agents Chemother 41:956–960
Badarau A, Shi Q, Chow JW, Zajicek J, Mobashery S, Vakulenko S (2008) Aminolgycoside 2″-phosphotransferase type IIIa from Enterococcus. J Biol Chem 283:7638–7647
Wright GD, Thompson PR (1999) Aminoglycoside phosphotransferase: proteins, structure, and mechanism. Front Biosci 4: D9–21
Shoichet BK (2004) Virtual screening of chemical libraries. Nature 432:862–865
Lin DL, Tran T, Adams C, Alam JY, Herron SR, Tolmasky ME (2013) Inhibitors of the aminoglycoside 6′-N-acetyltransferase type Ib [AAC(6′)-Ib] identified by in-silico molecular docking. Bioorg Med Chem Lett 23:5694–5698
Messaoudi A, Belguith H, Ben Hamida J (2013) Homology modeling and virtual screening approaches to identify potent inhibitors of VEB-1 β- lactamase. Theor Biol Med Model 22:1–10
Acknowledgments
KDS is gratefully acknowledged the University Grants Commission, New Delhi for financial support under UGC SAP Phase II programme [vide letter No. F. 4-8/2015/DRS-II (SAP-II)] sanctioned to Department of Biochemistry, Shivaji University, Kolhapur. RSP is thankful to UGC for providing BSR fellowship under UGC SAP DRS Phase I programme [vide letter No. F.7-207/2009 (BSR)]. SSB is grateful to DST PURSE-II scheme sanctioned to Shivaji University, Kolhapur, for providing fellowship. KDS is also thankful to DST SERB, New Delhi for providing infrastructural facility under project (Ref. No. EMR/2017/002688/BBM dated 25th October 2018). The authors are thankful to Department of Microbiology, Shivaji University, Kolhapur, for providing infrastructural facilities. Authors gratefully acknowledge to Department of Biotechnology, Shivaji University, Kolhapur for providing LCMS facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Responsible Editor: Jorge Luiz Mello Sampaio
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 11111 kb)
Rights and permissions
About this article
Cite this article
Parulekar, R.S., Barale, S.S. & Sonawane, K.D. Antibiotic resistance and inhibition mechanism of novel aminoglycoside phosphotransferase APH(5) from B. subtilis subsp. subtilis strain RK. Braz J Microbiol 50, 887–898 (2019). https://doi.org/10.1007/s42770-019-00132-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-019-00132-z