Abstract
In this paper, the absorption of CO and CO2 molecules on the two-dimensional structure of borophene has been investigated. Theoretical calculations based on density functional theory shows that the absorption energy of CO and CO2 molecules on borophene are much higher than that of graphene. Also, by lithium decorated borophene, the absorption energy is increased. So, it can be used as a toxic sensor of CO and CO2 gases. The monolayer borophene and borophene decorated with lithium is still conductive after absorption of CO and CO2 molecules. Furthermore, the I–V characteristics based on the non-equilibrium Green’s function calculations shows that currently, the limited effect occurs beyond 1.4 V and 2 V for the absorption of CO and CO2 molecules, respectively. Based on our findings, pure borophene can be used as the detector of CO and CO2 gas molecules, and decorated borophene with lithium can be used as an absorbent of these toxic gases from the environment.
Similar content being viewed by others
1 Introduction
Due to the danger of toxic gases on the health of living organisms, the production and development of toxic sensors and adsorbents are the investigation priorities of researchers [1, 2]. The excessive consumption of fossil fuels leads to the production of carbon oxides, such as CO and CO2 [3]. The identification and absorption of CO gas which is a colorless, odorless, tasteless, and toxic air pollutant and is produced in the incomplete combustion of carbon-containing fuels, extensively has been studied [4]. Also, the absorption of CO2 gas, which its concentration, led to the greenhouse effect in the Earth's atmosphere, has been studied [5]. In this regard, solid-state sensors are extremely regarded. Sensors based on semiconductor, metal–organic compounds [6], carbon nanotubes [7, 8], metal oxide nanowires [9] and two-dimensional compounds [10, 11] are among the latest subjects of this field.
Discovery of graphene and the characterization of its properties in 2004 [12] led to a revolution in the field of two-dimensional compounds. Due to the unique properties of graphene, such as very stable mechanical properties, electronic and transport features, it can have a high potential use in sensors and gas adsorbents [12]. Nevertheless, the absorption of CO and CO2 on this material is very weak and difficult to detect by the graphene sensor [13]. In 2015, Mannix et al. [14] succeeded in synthesizing a strange two-dimensional material from boron atoms called borophene. So far, various phases of this material have been experimentally synthesized [15, 16] and studied theoretically [15,16,17]. Previous studies have shown that borophene decorated with lithium can store up to 13.7 wt% of hydrogen [17]. This high absorption capacity has increased the incentive for studying the detection of various gases on this material. In addition, the unique electronic behaviors of borophene make it a suitable candidate for electronic applications. Calculations have shown that borophene is superconducting at 19 K, which also reaches 27.4 K by tensile strain [18]. Jiang et al. [19, 20] investigated the possibility of using borophene as anode for lithium-ion batteries. Theoretical capacity for lithium, sodium, and magnesium batteries is calculated as 1860, 1218, and 21,960 mAh/g, respectively [21]. Moreover, theoretical calculations have revealed borophene’s ability to be used as an electrocatalyst [22]. Borophene has many hydrogen adsorption sites that react easily for hydrogen evolution reaction (HER) with the lowest energy [22].
In this study, it is attempted to investigate the borophene as a unique two-dimensional structure of boron atoms as a toxic gas absorber of the CO and CO2. In this regard, by using the density functional theory (DFT), the adsorption of CO and CO2 molecules on the borophene is studied. Furthermore, the effect of decorating the borophene with lithium atoms on the absorption of CO and CO2 is also investigated. Subsequently, the electron transport and I–V curves corresponding to borophene decorated with lithium and without lithium are calculated to evaluate the sensor characteristics of this material. To accomplish this, the non-equilibrium Green’s function approach has been used before and after absorption of CO and CO2 gase.
2 Computational method
All calculations performed in this project are based on the density functional theory (DFT) utilizing the SIESTA package [23]. The exchange–correlation functional of the generalized gradient approximation (GGA) is framed by the Perdew–Burke–Ernzerhof approximation [24]. Also, Troullier–Martin type norm-conserving pseudopotentials [25] were employed in our calculations. First, in this calculation, the unit cell of borophene with two atoms is modeled (Fig. 1). The cut off energy is set to 50 Ry. The force between atoms is set smaller than 10–5 Ry/bohr. The wave function of atoms is extended via double-zeta polarized (DZP) basis set and the Monkhorst–Pack k-point mesh is adopted to 12 × 12 × 1. We have used the empirical dispersion-corrected DFT (DFT-D) method proposed by Grimme [26,27,28] to caclculate dispersion interactions more accurately. In order to investigate the absorption of CO and CO2 on this structure, after dimensional optimization and input parameters, the 3 × 3 supercell (Fig. 2) has been used in order to have sufficient spatial domain for the absorption of gases. This supercell has been constructed through the optimization of unit cell parameters. The interactions between two layers of borophene is extinct by using of 20 Å vacuum. Due to the existence of oxygen atoms in the structure, polarization is also included in the calculations. In addition, to investigate the properties of the borophene sensor to detect CO and CO2 gases, the current–voltage characteristics are calculated and computed using the TranSIESTA computational package. This computational package utilizes the non-equilibrium Green's function approach for calculating the electron transport of compounds. Lithium is also used to enhance the absorption capacity of borophene to absorb CO and CO2 molecules. When the borophene is decorated with lithium, then the number of absorption CO and CO2 molecules can be increased. The binding energy of borophene monolayer, CO and CO2 molecules are calculated as follows:
In this relation, \({E}_{b}\) is the binding energy, \({E}_{X+Borophene}\) is the total energy of the borophenesurface with the molecules absorbed on it, \({E}_{X}\) is the total energy of the CO and CO2 molecules, and \({E}_{Borophene}\) is the total energy of borophene in the absence of CO and CO2 molecules.
3 Results and discussions
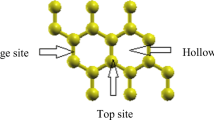
Borophene has tetragonal unit cell [17] as can be seen in Fig. 1. The lattice vectors a, b and the length of the B–B bond (Å) after optimization of the structure are obtained (Table 1) which are in a very good agreements with the previous reported results [14, 17, 29]. Table 1 demonstrates the B–B bond length and lattice vectors of borophene structure, compared with the previous theoretical and experimental data.
First, for studying the absorption of CO and CO2 molecules on a borophene the super-cell with size of 4 × 5 is constructed. Subsequently, CO and CO2 molecules are placed on different points on the borophene surface and their binding energies are calculated. Possible positions for CO molecules and five different places on the surface of the borophene are observed in Fig. 1. In the case, (1) the CO molecule is positioned perpendicular to the surface of the borophene from the end of oxygen atom, (2) the CO molecule is perpendicular to the borophene surface from the end of carbon atom (3) the CO molecule is parallel to the B–B bond and the B atom in the middle of the C–O bond; (4) the molecule of CO is parallel with the surface and perpendicular to the B–B bond; (5) the CO molecule is parallel to the surface and parallel to the B–B bond as well.
As shown in Table 2, the energy of the CO gas molecule in the position 4 is the strongest binding energy, and this position is the most stable position of the CO molecule on the borophene monolayer. This result indicates that the CO molecule prefers to be bonded to boron atoms in the borophene with the end of carbon atoms and that is a chemical absorption (see Fig. 2). This energy is stronger than the energy of the CO molecule on the graphene surface (− 0.05 eV [4]). In addition, the absorption energy of CO molecule in graphene in configuration (a) is − 0.01 eV that is approximately 35 times weaker than this for the monolayer borophane (− 0.352 eV). The absorption energy of CO molecule on the borophene from the end of O is − 0.352 eV which indicates that borophene weakly attractive the oxygen atom of the CO molecule.
In the following, we investigate the absorption of CO2 molecules on the surface of borophene. Similar to the CO molecule, five different configurations are considered (as can be seen in Fig. 4). These five configurations are: (1) the CO2 molecule perpendicular to the surface of the borophene; (2) the CO2 molecule parallel with the borophene surface and perpendicular to the B–B bonds; (3) the CO2 molecule parallel with the surface of the borophene and the upper layer of B–B bond as well; (4) the CO2 molecule parallel with the surface of the borophene and the lower layer of the B–B bond as well, (5) the CO2 molecule parallel with the borophene surface and perpendicular to the B–B bond, so that the C atom is located on top of the B atom.
The binding energy of the CO2 molecule on the borophene layer is calculated at five positions after absorption (Fig. 3). The binding energy of these configurations is obtained by using the Eq. (1) and their result is tabulated in Table 3. The most stable position of the CO2 molecule on the borophene is the configuration of 5 with an absorption energy of − 0.651 eV, indicating a physical absorption. The absorption energy of CO2 molecule on the borophene is approximately 14 times larger than its absorption energy on the graphene (− 0.047 eV [30]). Thus, the borophene is stronger absorber for CO2 adsorbent than the graphene. The most stable position of the CO2 molecule on the surface of the borophene is shown in Fig. 4. The values reported in Table 3 clearly show that CO2 absorption on the surface of the borophene is a physical absorption and a strong chemical bond between the CO2 molecule and the borophene surface is not formed. However, the energy related to the most stable position of the CO molecule is almost 4 times (− 2.125 eV) larger than the steady state of absorption of the CO2 molecule (− 0.651 eV) on borophene.
After finding the most stable position of CO and CO2 molecules on the surface of borophene, the effect of this adsorption on the electronic properties of borophene is investigated. In Fig. 5, the band structure and density of states corresponding to the pure borophene structure and in the presence of CO and CO2 molecules are plotted. As shown in Fig. 5a, the borophene is conductive and has no energy gap. The calculated band structure of pure borophene has good agreement with the previous works [15]. Figures 5b, c also show that with the addition of CO and CO2 molecules on the borophene, the bands cross the Fermi level and the structures behave as metal material.
In the following, the absorption of CO and CO2 molecules in the decorated borophene with lithium atoms is investigated. In order to find the best absorption position of the lithium atom on the surface of the borophene, the lithium atom is located in the positions of 1, 2, 3, and 4 (Fig. 6a). After the structure’s relaxation, their binding energy is calculated according to Eq. (1). The positions of Li atom including (1) on the boron atom, (2) on the upper B–B bond, (3) in the middle of lower B–B bond (4) in the middle of triangle formed from boron atoms are investigated. According to the results obtained (Table 4), the best position of absorption of lithium atom is at position 4, with the binding energy of − 1.791 eV, which is much larger than the lithium cohesive energy in the lithium-bulk structure (− 1.6 eV) [31]. The larger binding energy of the lithium atom on the surface of the borophene compare to its cohesive energy in the bulk structure shows that the lithium atom without transformed to multi-atomic lithium cluster on the surface of the borophene can be completely separate and single-atomic distributed. Since lithium atom have low electronegativity, it has tendency to lose a single electron in their valence band. So, after lithium absorption, the borophene structure remain conductive and with no band gap.
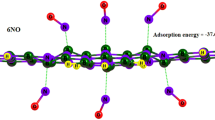
After decorating the borophene with the lithium atom, it has been attempted to absorb CO and CO2 molecules on its surface. First, one CO/CO2 molecule is located on the decorated borophene with the lithium atom, and then by increasing the number of molecules, the maximum number of CO/CO2 molecule that can be absorbed on the decorated borophene is obtained. In Table 5, the absorption energies are given. The results of Table 5 show that at most two CO/CO2 molecules can be absorbed on the lithium-decorated monolayer borophene. Our results indicate that lithium atom increases the absorption energy of CO2 (− 1.155 eV) molecules.
In order to explore origin of the adsorption strength of these gases on Li-decorated borophene, a Hirschfield charge analysis was performed, the results are listed in Table 5. The amount of electrons transferred from CO to Li-decorated borophene is the largest about 0.18e, which leads to the maximum adsorption energy (Fig. 7).
After finding the most stable absorption of CO/CO2 molecules on lithium-decorated borophene, the effect of this absorption on the electronic structure is studied. For this purpose, the band structure and density of states of these structures are depicted in Fig. 8. As it can be seen, these structures remain conductive and no band gap was observed for them.
4 CO and CO2 gas sensors
In order to design the CO and CO2 gas sensor, the effect of absorbing of these gases on the current–voltages curves should be calculated. The I–V characteristic of monolayer borophene before and after absorption of CO and CO2 molecules are obtained. The geometry of the designed device (Fig. 9) consists of a central region as a scattering, and the left and right electrodes are shown in the Fig. 10. Electrodes are considered as an electron gas model with a specific chemical potential. The electron transport properties studied in the Z-direction with a mesh size of 1 × 1 × 50. The length of the scattering region is at least 12 Å to prevent possible interference of the electrodes.
Figure 10a shows the I–V curves of the pure monolayer borophene. In this Figure with increasing voltage from 0.0 to 1.4 V, the current increases with constant gradients, but after 1.4 V, the current remains constant at 150 μA and does not change much with increasing the bias voltage. In Fig. 10c, e, the I–V characteristic of monolayer borophene flow after absorption of CO and CO2 molecules are shown. In these two graphs, after the absorption of the gas molecule, by increasing the voltage, the current increases with a constant gradient. But this trend of increasing the current by increasing the voltage bias, as well as I–V curves of pure borophene, have stopped beyond the 1.4 V and their current becomes almost constant. The current of absorbing CO and CO2 molecules on borophene reaches 75 ± 10 μA and 95 ± 10 μA, respectively, beyond the 1.4 V. Since CO2 is absorbed physically on the surface of the borophene, it has a weaker interaction with monolayer. The weaker interaction of CO2 molecules compared to the CO molecules with the monolayer borophene has led to a smaller drop in the current of CO2 molecule than in the other molecule. Furthermore, there is a difference equal to 20 μA between the pure borophene and the absorbing CO and CO2 molecules on the borophene at the voltages higher than 1.4 V, which can help to differentiate these two gases in designing the sensors for better detection.
In Fig. 10, the I–V characteristic of Li-decorated borophene is shown before and after absorption of CO and CO2 gas molecules. Unlike pure borophene, the current of Li-decorated borophene after the voltage of 1.4 V, continues to increase with a slight gradient. Also, after absorption of CO and CO2 molecules, the current is more intense and the current rate increases with increasing voltage. Figure 10b shows that Li-decorated borophene has a higher current rate than a pure borophene. The smaller lithium electronegativity (0.98) compared to boron (2.04) leads to the transfer of charge from lithium to the borophene monolayer. As a result, the current density increases and the current rate increases even more than the pure borophene. Unlike pure borophene, Li-decorated borophene exhibits a higher current after absorption of CO molecules than that of CO2 absorption. Upon absorption of CO on Li-decorated borophene, the current has increased to 100 μA under bias voltage of 2.0 V. However, after absorbing CO2, the current has reached a maximum of 85 μA. The current–voltage effect has not been observed for these configurations with increasing voltage, which cannot be used to distinguish between the absorption of CO and CO2 molecules along with sensor application.
5 Conclusions
The results of the calculations show that borophene is a stronger absorber than the graphene in absorption of CO and CO2 molecules. The absorption energy of CO molecules on borophene is 2.075 eV larger than graphene. Furthermore, the absorption energy of CO2 molecules on borophene is 14 times stronger than that of graphene. Therefore, borophene can be a powerful absorbent of carbon oxides. However, CO2 molecules are physically absorbed on the surface of the borophene, through the decoration of borophene with the lithium atoms, the absorption of CO2 molecules is chemical. Therefore, Li-decorated borophene can be excellent absorbent for CO and CO2 toxic gas molecules. Studies on the borophene device showed that pure borophene could detect CO and CO2 gases for voltages above 1.4 V with a constant current. This feature can be very useful in designing highly sensitive sensors that can easily distinguish CO and CO2 from one another. In contrast, Li-decorated borophene has a higher current than a pure borophene. The smaller lithium electronegativity than the boron leads to the charge transfer of lithium from borophen monolayer, resulting in a higher current flow density than the pure borophene and a higher current rate. However, the trend of increasing the current with increasing voltage after absorption of CO and CO2 molecules on decorated borophene is not very reliable for designing the sensor for detecting these gases.
References
Kong J, Franklin NR, Zhou C, Chapline MG, Peng S, Cho K, Dai H (2000) Nanotube molecular wires as chemical sensors. Science 287(5453):622–625
Faghihnasiri M, Rezvani M, Shabani M, Firouzian AH (2016) The temperature effect on mechanical properties of silicon carbide sheet based on density functional treatment. Solid State Commun 227:40–44
Lee K-J, Kim S-J (2013) Theoretical investigation of CO2 adsorption on graphene. Bull Korean Chem Soc 34(10):3022–3026
Wanno B, Tabtimsai C (2014) A DFT investigation of CO adsorption on VIIIB transition metal-doped graphene sheets. Superlattices Microstruct 67:110–117
R. F. Service (2004) The carbon conundrum. Science (New York, NY) 305(5686):962
Li J-R, Kuppler RJ, Zhou H-C (2009) Selective gas adsorption and separation in metal–organic frameworks. Chem Soc Rev 38(5):1477–1504
Azizi K, Hashemianzadeh SM, Bahramifar S (2011) Density functional theory study of carbon monoxide adsorption on the inside and outside of the armchair single-walled carbon nanotubes. Curr Appl Phys 11(3):776–782
Ansari R, Malakpour S, Faghihnasiri M, Ajori S (2013) Structural and elastic properties of carbon nanotubes containing Fe atoms using first principles. Superlattices Microstruct 64:220–226
Zhang D, Liu Z, Li C, Tang T, Liu X, Han S, Lei B, Zhou C (2004) Detection of NO2 down to ppb levels using individual and multiple In2O3 nanowire devices. Nano Lett 4(10):1919–1924
Radovic LR (2005) The mechanism of CO2 chemisorption on zigzag carbon active sites: a computational chemistry study. Carbon 43(5):907–915
Ahmadi A, Jafari H, Rajipour M, Fattahi R, Faghihnasiri M (2019) Nonlinear electronic transport behavior of Υ-graphyne nanotubes. IEEE Trans Electron Devices 66(3):1584–1590
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306(5696):666–669
Ao ZM, Yang J, Li S, Jiang Q (2008) Enhancement of CO detection in Al doped graphene. Chem Phys Lett 461(4–6):276–279
Mannix AJ, Zhou X-F, Kiraly B, Wood JD, Alducin D, Myers BD, Liu X, Fisher BL, Santiago U, Guest JR (2015) Synthesis of borophenes: anisotropic, two-dimensional boron polymorphs. Science 350(6267):1513–1516
Faghihnasiri M, Jafari H, Ramazani A, Shabani M, Estalaki SM, Larson RG (2019) Nonlinear elastic behavior and anisotropic electronic properties of two-dimensional borophene. J Appl Phys 125(14):145107
Vishkayi SI, Tagani MB (2017) Current–voltage characteristics of borophene and borophane sheets. Phys Chem Chem Phys 19(32):21461–21466
Ji Y, Dong H, Li Y (2017) Theoretical predictions on Li–decorated borophenes as promising hydrogen storage materials. ChemistrySelect 2(31):10304–10309
Li G, Zhao Y, Zeng S, Zulfiqar M, Ni J (2018) Strain effect on the superconductivity in borophenes. J Phys Chem C 122(29):16916–16924
Jiang HR, Lu Z, Wu MC, Ciucci F, Zhao TS (2016) Borophene: a promising anode material offering high specific capacity and high rate capability for lithium-ion batteries. Nano Energy 23:97–104
Shi L, Zhao T, Xu A, Xu J (2016) Ab initio prediction of borophene as an extraordinary anode material exhibiting ultrafast directional sodium diffusion for sodium-based batteries. Sci Bull 61(14):1138–1144
Mortazavi B, Dianat A, Rahaman O, Cuniberti G, Rabczuk T (2016) Borophene as an anode material for Ca, Mg, Na or Li ion storage: a first-principle study. J Power Sources 329:456–461
Shi L, Ling C, Ouyang Y, Wang J (2017) High intrinsic catalytic activity of two-dimensional boron monolayers for the hydrogen evolution reaction. Nanoscale 9(2):533–537
Soler JM, Artacho E, Gale JD, García A, Junquera J, Ordejón P, Sánchez-Portal D (2002) The SIESTA method for ab initio order-N materials simulation. J Phys Condens Matter 14(11):2745
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865
Troullier N, Martins JL (1991) Efficient pseudopotentials for plane-wave calculations. Phys Rev B 43(3):1993
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27(15):1787–1799
Grimme S (2004) Accurate description of van der Waals complexes by density functional theory including empirical corrections. J Comput Chem 25(12):1463–1473
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132(15):154104
Peng B, Zhang H, Shao H, Xu Y, Zhang R, Zhu H (2016) The electronic, optical, and thermodynamic properties of borophene from first-principles calculations. J Mater Chem C 4(16):3592–3598
Noei M (2016) DFT study on the sensitivity of open edge graphene toward CO2 gas. Vacuum 131:194–200
Liu C-S, Zeng Z (2010) Boron-tuned bonding mechanism of Li-graphene complex for reversible hydrogen storage. Appl Phys Lett 96(12):123101
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest and have participated in (a) conception and design, or analysis and interpretation of the data; (b) drafting the article or revising it critically for important intellectual content; and (c) approval of the final version.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arefi, V., Horri, A. & Tavakoli, M.B. CO/CO2 adsorption and sensing on borophene. SN Appl. Sci. 2, 1304 (2020). https://doi.org/10.1007/s42452-020-3114-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-3114-4