Abstract
This study presents a controlled sacrificial anode (magnesium anodes) cathodic protection system for aboveground storage tanks. The proposed method is able to control the anode’s current, which leads to enhance the performance of anodes, therefore, increase the anode’s lifetime. The proposed system has been implemented in a laboratory-based tank contains saline water (5661 ppm). In this experiment, the overprotection and anode energy loss were eliminated, and the anodes lifetime has been extended 35.55 times higher compared to the conventional system. The proposed method reduced the protection current and stabilized the system overall.
Similar content being viewed by others
1 Introduction
The corrosion process is an oxidation–reduction reaction concept where electrons are removed from a metal surface and replace them with oxygen from the surrounding environment [1, 2]. The conventional method to prevent metal corrosion is by coating or painting the surface of the metal. Corrosion causes waste of valuable resources, loss or impurity of productivity, efficiency, reduction, and often plant shutdowns. The corrosion can also inhibit technological progress and jeopardize safety. In corrosion control of metallic structures, the main issue with the conventional sacrificial anode system is the excessive, uncontrollable current flow by the anodes, which leads to high current flow to the storage tank and causing overprotection, coating defect, and short anode’s lifetime.
The coating method is widely used to isolate the metals from contact with the surrounding electrolytes, such as water and soil. However, the coating cannot provide full protection; thereby, corrosion will take place at the breaks in the coating [3]. The cathodic protection (CP) is one of the most effective and economical technique to prevent structural corrosion by making the metal surface as a cathode of an electrochemical cell [4]. The cathodic protection system can be applied to any metallic structure such as tanks, buried pipelines, ships, marine’s jetties, and any metals that have contact with electrolytes such as soil or water [5]. The CP system can be performed either by injecting current cathodic protection (ICCP) or sacrificial anode (SA) [6]. The ICCP system requires an external DC power source that is connected to the structure by using particular types of anodes, while CP with SA does not require any external power source. The former one is more suitable for the protection of larger structures like long pipe systems, while the latter one is more suitable for the protection of smaller structures such as tanks [7], which is the interest of this study. The sacrificial anode is a metal that has a more negative charge than the body of the structure. In conventional SA systems of structures in the sea and freshwater, the anodes are directly connected by welding to the body of the structure.
Olusunle et al. [8] investigated the effects of painted structures on SA system performance. Their study suggested that the sacrificial anode system can result in an economical and higher performance in terms of a longer anode lifetime if the metallic structure is coated instead of the bare structure [8].
Ekhasomhi et al. [9] conducted a case study for designing an SA-based CP system to protect the internal face bottom and internal shell of barrels (BBLS) crude oil surge tank. In this study, zinc anodes have been used as an SA to protect a commercial tank which was made of mild steel material. The aim of this study was to determine if the zinc sacrificial anode system was an acceptable and convenient solution to control corrosion .
Magnesium, aluminium, and zinc are the metals that are commonly used as SA [10]. Zinc anodes are used when the soil resistivity is less than 20 mΩ, and Zinc and aluminium anodes are used in seawater. Technically, magnesium would be the best material as SA due to its higher potential. However, this higher potential causes overprotection and generates the development of hydrogen gas, which may cause coating defect and coating disbondment from the tank [11]. In addition, due to the high current flow, the anodes will be exhausted in a short time. The conventional SA-based CP system suffers from the lack of having a controllable current/potential system. This issue encourages to have an SA system which its current and potential can be controlled [12].
Nustad et al. [13] have proposed a fixed built-in resistor to reduce the potential of the pipeline structures. In this method, a fixed resistor was embedded inside in series with the anode to drop the existing charges [13]. However, this method did not have the flexibility to control the optimum current flow required in a CP system. The optimum current flow may need to be changed due to the change of resistance of the electrolyte, material surface polarization, and/or corrosion status of the structure.
Narozny et al. [11] have proposed a transistor-driven SA system implemented on a laboratory steel structure that was placed in seawater. A PNP transistor was used to control the current of the magnesium anode [11]. However, the proposed system was limited only to the metallic structures that have very low potential compared to the potential of the anode. Also, it required an additional anode as an auxiliary electrode to be connected to the base of the transistor.
The sacrificial anode system is the most common solution for protecting structures from corrosion [14]. The Mg is usually used as a sacrificial anode to provide cathodic protection of underground and aboveground structures such as ships, tanks, and pipelines [15].
In this paper, a fully controllable SA system is proposed using a variable resistor for above ground tank structures. It is shown that by optimizing the current flow of the structure, the magnesium anodes’ lifetime can be significantly improved compared to the conventional SAs.
2 Materials and method
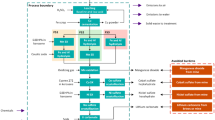
Figure 1 shows the schematic diagram of the proposed design SA CP system. A variable resistor was proposed to be in series with the anodes where all anodes were connected in parallel and isolated from the tank body using insulator pillars to avoid a direct connection to the tank body.
In this study, three identical tanks with dimensions of 20 cm diameter and 22 cm height made of similar material were developed and coated with two layers of Samurai epoxy in order to evaluate the performance of the proposed SA system. Ribbon Magnesium anodes were used in this study as the anodic material. For all three tanks, similar anode dimensions and installation structures were applied. The dimensions of the magnesium ribbon anodes were calculated based on the consumption rate equation of the Conventional SA system for the duration of 1 month based on a specific electrolyte of saline water with a TDS (Total Dissolved Solids) of 5661 ppm as shown in Table 1 [16].
The most important part of designing a cathodic protection system is the required current per square meter which is called (current density) to shift the structure’s potential to a minimum − 0.85 V with respect to the copper/copper sulphate reference electrode. The required current can be calculated as follows [17].
where It is the total current required in amperes, i is the current density required in a unit of A/m2, and A is the surface area to be protected in a square meter, which can be either for internal or external surface area. Thereby, the weight of ribbon required for internal surface bottom or tank shell can be calculated as follows [17]:
where W is the weight of ribbon anode, Y is CP system lifetime, and I is the amount of current required for internal tank bottom or tank shell, Z is the capacity of alloy, and U is anode utilization factor.
The length of ribbon required can be calculated as follows [17]:
where the L is the length of the ribbon anode required, W is the total weight, and w is the weight/meter of anode ribbon.
The anode coverage for both internal and external can be calculated as follows [17]:
where S is the ribbon anode separation in meter, d is the distance between tank structure, and ribbon anode is in meter.
Therefore, the required number of anodes for internal and external protection can be calculated as follows [17]:
where N is the number of the ribbon anodes in parallel, D is the tank diameter in meter and S is ribbon anode separation in meter.
The saline water was made up by mixing one litre of fresh water and 11 g of sea salt. This electrolyte was used for all experiments in this study. A commercial TDS (Total Dissolved Solids) meter model EcoHydro was used to monitor the TDS of the saline in the tanks on a daily basis during the experiments to make sure the TDS level is not changed. In case of any change in the water TDS, the water was changed to keep the TDS constant. The salinity of the used water is 5661 ppm.
Out of the three tanks, one tank (Tank-1) has been used for implementing the conventional SA system. Figure 2 illustrates the magnesium anodes, which initially were welded to the internal tank shell and internal tank bottom before the tank was painted. The performance of this tank is used as the benchmark to compare with the performance of the proposed design.
Since the proposed system shown in Fig. 1 requires the anodes to be isolated from the tank body, it is the interest of this study to evaluate the influence of this isolation on the corrosion protection as well as anode lifetime before the variable resistor is applied in the system. Thereby, the second tank (Tank-2) was used to implement a system similar to the proposed design in Fig. 1, without the variable resistor as the schematic diagram is illustrated in Fig. 3. The “ON” potential measured when the switch S1 is closed while the “OFF” potential will be measured when the switch S1 is open.
In addition, the current flow of the tank was measured by connecting an ammeter in series with the circuit, as shown in Fig. 3. It should be noted that the current flows through the circuit only when the circuit is in “ON” position. The “OFF” state is only for a short time to measure the “OFF” potential of the tank. In this condition, there is no current flow through the circuit when the switch is opened.
The third tank (Tank-3) was used to implement the complete proposed design, as shown in Fig. 1. The switch S1 is placed in series in the system to allow “ON” and “OFF” potential measurements. An ammeter has been used to measure the protection current in this project, as shown in Figs. 1 and 3.
Figure 4 shows a schematic diagram of how the anodes can be isolated from the tank body. The busbar isolator shown in Fig. 4 is a commercially available isolator that can be used for isolating anodes from the tank body as required in the proposed Tank-2 and Tank-3. One side of the busbar isolator can be welded to the tank body, while the other side of the bus bar isolator can be fixed into the holder box, as shown in Fig. 4. It should be noted that the two sides of the busbar isolator are not electrically connected.
3 Results and discussion
After fabrication of the three tanks, the potential of the bare steel tanks before coating and installing anodes, have been measured. The initial potentials were measured in the range of − 0. 550 to − 0. 553 V with respect to the copper/copper sulphate reference electrode. This potential is in agreement with the potential of the clean and shiny mild steel reported to be in the range of − 0. 5 to − 0. 8 V with respect to copper/copper sulphate reference electrode [18]. Since the potential values are lower than the minimum protection voltage, − 0. 850 V [19], the bare tank is expected to be corroded due to the environmental condition such as water, soil and humidity.
The potential of the Tank-2 and Tank-3 have been measured again after the tanks were coated, which showed around − 0. 606 to − 0. 608 V. This result shows that the protection level is increased compared to the bare tank, but it is still not enough for self-protection from corrosion, based on the CP potential criteria.
3.1 Performance analysis of Tank-1
As stated in Sect. 2, Tank-1 was used for implementing the conventional SA protection system for the purpose of benchmarking and performance comparison with the proposed system. The potential of the internal tank has been measured on a daily basis by using copper/copper sulphate reference electrode until the anodes are fully consumed, as shown in Fig. 5. The electrode was placed in the centre of the tank’s bottom to measure the potential of the tank’s bottom and to measure the potential of the tank’s wall, and the electrode was placed near the tank’s wall. It was noticed that the two readings from the mentioned set up were almost equal because of the small size of the prototype and also due to a low seawater resistivity. The low resistivity of the electrolyte made the potential drop (IR) neglected. Therefore, the copper/copper sulphate reference electrode was placed in the centre of the tank’s bottom to measure the potential of the wall and the tank’s bottom as well. The copper/copper sulphate reference electrode was used for a short time during the measurement only.
Figure 6a and b show the size of the original anode and the consumed anode taken out from the Tank-1, respectively. The results for the internal tank potential for “ON” condition shows around − 1. 6 V for the first 20 days and then started to reduce gradually till the day 32 that the potential was already reduced to − 0. 7 V. This was lower than the minimum protection potential level, as illustrated in Fig. 5. In this case, the lifetime of the conventional SA system lasts for only about 31 days based on the experimental results, which is almost closed to the theoretical one-month design lifetime. The reason for the short lifetime of the conventional SA system is due to the high current delivered by the anodes. The current of the conventional SA system was not possible to be measured as the anodes were welded to the tank body. The high current delivered by the anodes not only affected the lifetime of the CP system but also causes coating defects. Based on our observation, the coating of the internal tank was in good condition for the first 15 days, but after that, the coatings started to defect in the form of disbonding from the tank body in some parts of the internal tank. A few days later, between days 18-31, the coating has been cracked in the disbanded area and eventually toward the end of the experiment which part of the coating was separated from the tank body.
3.2 Performance analysis of Tank-2
The design depicted in Fig. 3 was implemented in Tank-2, and it was observed until the anodes were fully consumed. The potential of the internal tank has been measured by using copper/copper sulphate reference electrode every day, and the achieved result is shown in Fig. 7. The result shows that for the first 22 days, the “ON” potential was relatively constant around − 1.616 V and gradually reduced to − 1.4 V. On the 48th day, it was observed that the potential level was rapidly reduced while on the 53rd day, its potential level measured below the minimum protection level, − 0.731 V as illustrated in Fig. 7. The “OFF” condition potential was measured in between − 1.2 to − 1.3 V, as shown in Fig. 6.
On the other hand, the results of current flow show that the current level for the first 6 days was high about 80 mA before the polarization of the tank body. Then, the current reduced to around 60 mA at 11th day, and again, it dropped to about 35 mA in 31st day. After that, the current gradually reduced to about 22 mA on the day 47th and then it is reduced to about zero on the 53rd day. The drop of the potential from the day 50–53 was due to the anodes which have been fully consumed, and the image of the consumed anode is shown in Fig. 6c.
The results and observations of this experiment show that the potential level in the Tank-2 was also considered as overprotection compared to the minimum potential, − 0.85 V required for protecting the tank. Thereby, this overprotection causes the high current consumption rate of anodes and, thereby, leads to a short anode lifetime around 52 days.
3.3 Performance analysis of proposed design Tank-3
In this experiment, the proposed design depicted in Fig. 1 was implemented in Tank-3. The “ON” and “OFF” potentials and current of the internal tank have been measured by using copper/copper sulphate reference electrode and digital multimeter, respectively and the results are shown in Fig. 8. In this experiment, the current/potential of the tank body has been controlled by using a variable resistor to achieve the minimum protection potential and thereby minimum current to meet the CP criteria. In this study, the potential of the tank was set to − 1. 22 V, slightly higher than the minimum required potential to allow the tank body to be polarized. Based on this potential, the initial current was measured to be around 4.2 mA for the first 12 days and then after the tank body is slightly polarized the current flow decreased to 4 mA and then remained almost constant until the 33 days. The results suggest that in the 33rd, a full polarization of the tank body has occurred. At a current of 4 mA noticed that the “OFF” potential being increased so, the current has been adjusted to 1 mA in order to avoid the overprotection and then the current remained constant until the experiment has been stopped on the day 59th due to achievement of full polarization. Figure 6d shows the image of one example of the anodes taken out from the Tank-3 after the 59th day in comparison with the original anode shown in Fig. 6a.
Comparing the results of Tank-1 and Tank-2, it can be noted that by insulating the anodes from the tank body, the anodes lifetime can be extended to 1.67 times compared to the conventional SA where the anodes were welded to the tank body.
Comparing the performance of the setup in Tank-2 with Tank-3 it can be noticed that the isolation of the anodes without controlling the current flow does not contribute a significant extension to the anode lifetime. To further compare the performance of the three tanks, two methods were considered here, one based on the current flow and the second based on the weight of the anodes. For the first comparison, since the experimental current of the conventional SA in Tank-1 is not available, it has been calculated to be 142.2 mA based on the experimental values. The consumption rate of the anode has been calculated from the proposed design in Tank-2 based on the anode’s current output and its lifetime. The current for the Tank-1 conventional CP system has been calculated and considering the initial current draw of 4 mA in Tank-3 compared to the conventional SA. The Tank-1 shows that the proposed SA system can improve the life expectancy of the anode about 35.55 times longer. On the other hand, comparing the remaining weight of the anodes in the Tank-3 which was 31 g, to the original weight of anodes 33 g, it shows the outperformance of the proposed setup in Tank-3 by 31.4 times, which is in agreement with the results shown above is based on the current consumption.
4 Conclusion
The aim of this study was to improve the performance of the SA protection system. The application of a resistor-controlled design reduced the required current output, which is to protect the internal shell and tank bottom in saline water. The proposed design (Tank-3) is to isolate the anodes from the tank body and utilizes a variable resistor to allow full control of the tank potential/current. The proposed design has been examined experimentally and the performance was compared with the conventional SA system. The proposed design (Tank-3) has shown that the anode life expectancy improved 35.55 times (based on the current) and 31.4 times (based on anode weight) compared to the conventional SA systems. The improvement of a sacrificial anode lifetime increased up to 35.55 times, which was a significant accomplishment that never has been reported before for cathodic protection of tank applications. Also, in the proposed design, the overprotection and anode energy loss were eliminated. Thereby, by implementing the proposed design (Tank-3), the performance of the anode has been enhanced compared with the conventional SA systems. Besides the lifecycle improvement, the proposed design (Tank-3) offers other advantages such as no coating defect as well as easier anode installation and maintenance, which the anodes do not need to be welded to the tank body which they can be easily fixed. The proposed method in this study would be very helpful for industries associated with storage tanks for storing water, cooking oil, industrial oil, and gas industries.
In order to improve the life expectancy of the system, it is recommended that aluminium or zinc anodes to be considered beside magnesium anode for future work.
References
Kumar R, Dwivedi S (2014) Corrosion in underground tank and its prevention by cathodic protection process. Int J Basic Appl Sci 4(4):51–58
Shehadeh M, Hassan I (2013) Study of sacrificial cathodic protection on marine structures in the sea and fresh water in relation to flow conditions. Ships Offshore Struct 8(1):102–110. https://doi.org/10.1080/17445302.2011.590694
CP1—Cathodic Protection Tester (2012) NACE International, Houston
Guma TN, Mohammed SU, Tanimu AJ (2016) An experimental investigation of galvanic anode specifications for suitable cathodic corrosion protection of low carbon steel in Kaduna metropolitan soil. Am J Eng Res 5(2):109–119
Riemer D, Orazem M (2005) A mathematical model for the cathodic protection of tank bottoms. Corros Sci 47(3):849–868
Narozny M, Zakowski K, Darowicki K (2017) Time evolution of electrochemical impedance spectra of cathodically protected steel in artificial seawater. Constr Build Mater 154:88–94. https://doi.org/10.1016/j.conbuildmat.2017.07.191
Gurrappa I (2005) Cathodic protection of cooling water systems and selection of appropriate materials. J Mater Process Technol 166(2):256–267. https://doi.org/10.1016/j.jmatprotec.2004.09.074
Olusunle SOO, Ogundare OD, Akinribide OJ, Adebo GJ (2015) Use of sacrificial anode for corrosion protection of tradition well cover. Int J Eng Technol 5(5):269–274
Ekhasomhi A, Ehisuan Y, Ariavie G (2017) Design of a cathodic protection system for 2,000 barrels crude oil surge tank using zinc anode. J Multidiscip Eng Sci Technol 4(3):6905–6908
Peabody A, Bianchetti R (2001) Peabody’s control of pipeline corrosion, 2nd edn. NACE International, The Corrosion Society, Houston, pp 177–184
Narozny M, Zakowski K, Darowicki K (2014) Method of sacrificial anode transistor-driving in a cathodic protection system. Corros Sci 88:275–279. https://doi.org/10.1016/j.corsci.2014.07.041
Peabody A, Bianchetti R (2001) Peabody’s control of pipeline corrosion, 2nd edn. NACE International, The Corrosion Society, Houston, p 28
Nustad G et al (2003) Resistor controlled cathodic protection for stainless steels in chlorinated seawater a review after 8 years in-service. NACE Int 1–15
Radwan A, Elbatran A, Mehanna A, Shehadeh M (2017) Experimental study on using the aluminium sacrificial anode as a cathodic protection for marine structures. Int J Mar Environ Sci 11(1):73–77
Pathak S, Mendon S, Blanton M, Rawlins J (2012) Magnesium-based sacrificial anode cathodic protection coatings (Mg-rich primers) for aluminum alloys. Metals 2(3):353–376. https://doi.org/10.3390/met2030353
Nielsen D, Brock M, Rees G, Baldwin D (2003) Effects of increasing salinity on freshwater ecosystems in Australia. Aust J Bot 51(6):655–665. https://doi.org/10.1071/bt02115
CP4—Cathodic Protection Tester Course Manual (2012) NACE International, Houston
Peabody A, Bianchetti R (2001) Peabody’s control of pipeline corrosion, 2nd edn. NACE International, The Corrosion Society, Houston, p 21
Peabody A, Bianchetti R (2001) Peabody’s control of pipeline corrosion, 2nd edn. NACE International, The Corrosion Society, Houston, pp 49–55
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jawad, M.N., Amouzad Mahdiraji, G. & Hajibeigy, M.T. Performance improvement of sacrificial anode cathodic protection system for above ground storage tank. SN Appl. Sci. 2, 1979 (2020). https://doi.org/10.1007/s42452-020-03823-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03823-7