Abstract
The photoconductivity within a wavelength range of 450–1100 nm was determined for a sample of epitaxial delafossite CuFeO2 film grown by pulsed laser deposition. The film thickness was estimated to be 75 nm. The resistance of the films was determined with four-contact van der Pauw’s method and using monochromatic illumination of the film. The most significant change in resistance resulted in three rapid lineal conductivity increases at photon energies of ~ 1.5 eV (gap-1), ~ 2.1 eV (gap-2) and ~ 2.5 eV (gap-3). The conductivity properties are well correlated with prior optical absorption results obtained in the NIR-VIS region using transmittance spectroscopy.
Similar content being viewed by others
1 Introduction
The global population has increased almost eighth-fold in the last 200 years. Consequently the demand for energy has also increased exponentially. Between the years 2005 and 2030 the demand for energy will increase by 55% the energy used at the beginning of that period of time [1]. Most of the energy being used now comes from fossil fuels but the problems related to burning fossil fuels are a driving motor to search for new renewable energy sources [2].
The combustion of fossil fuels yields emissions of gases such as carbon dioxide and methane which are responsible for the “greenhouse effect” in our planet and consequently the undesirable global warming.
Hydrogen is an attractive renewable energy source and seems to be a good alternative to energy obtained from fossil fuels combustion [3,4,5]. Hydrogen can be obtained from water electrolysis and in order to make this process more energetically efficient, the possibility of using solar radiation in a photo-catalytic molecular decomposition of water is desirable and requires a multidisciplinary study.
Water dissociation by electrochemical photolysis was pioneered by Fujishima and Honda [6] using TiO2 photo-electrodes.
Other widely reported electrode materials used for water splitting were WO3, CdS and other varieties of multi-component compounds [7, 8] which were broadly reviewed by Maeda [9]. However, research continues on chemically stable materials with a band gap greater than the water splitting potential of 1.23 V. Such materials should also display strong band to band absorption characteristics corresponding to peak solar irradiance. It has been predicted that such materials would enhance the efficiency of the photo-catalytic water decomposition [10].
Sivula and Van de Krol [11] drew a diagram of several semiconducting materials and their band gap (valence and conduction edge) overlapping with the oxidation and reduction potential for water dissociation. The oxidation (production of O2) and reduction (production of H2) potentials move according to the electrolyte used in the water. The most appropriate materials for photolysis are those whose valence and conduction edge (separated by band gap) fit within these two values of potential which are distant 1.23 V from each other.
The value of 1.23 V comes from thermodynamics equilibrium for the following chemical reaction:
The non-catalyzed chemical reaction needs 1.23 eV to break each O–H bond on the left side of the equation (there are four O–H bonds).
Therefore, this value of 1.23 V for the potentials separation is only an estimate since the dissociation of water is catalyzed by the surface of the photoconductor.
Several materials shown in Sivula and Van de Krol [11] diagram draw special attention: TiO2 is the first semiconductor tested in water photolysis; CuFeO2 with delafossite crystal structure; and a BiVO4, recently reviewed [12], indicates that these materials have a lot of potential for photolysis.
One promising type of materials for these applications is CuFeO2 and the whole family of oxides with a delafossite crystal structure. These compounds are characterized by the general chemical formula ABO2, where A is a monovalent cation and B is a trivalent metal, any from Al to La [13]. This oxide family also displays multi-band-gap semiconductor behavior with energy gaps ranging between 1.1 and 3.6 eV and shows band-to-band absorption characteristics in response to illumination by UV, visible, and near infrared light. One advantage over the most common semiconductor materials is that the band gap(s) of delafossite oxides can be adjusted by changing the composition of the oxide. An additional advantage of these complex oxides is the abundance of the elements used in their composition such as Cu, Fe, Al, etc.
The CuFeO2 delafossite oxide is known to be a p-type semiconductor [13]. However, reports of the fundamental energy gap vary widely. There are reports of multiple dominant absorption edges forming step-like patterns in the optical spectra of delafossite CuFeO2 which are thought to emerge from the superposing of CuO2 and Fe2O3 absorbance properties within the composition of delafossite CuFeO2 [14]. Additionally, the breaking up of dipole-forbidden transition mechanisms is thought to emerge from Fe-3d states positioned within the forbidden regions of the crystalline structure [15].
Band gap measurements of CuFeO2 samples have yielded scattered results within a wide range of energy. The difference in the band gap values could be explained by the fact that samples synthesis methods are all different: Pulsed Laser Deposition; sol–gel method; and solid state reaction. Additionally, the material itself could be a multiple-band gap semiconductor.
The band gap results for CuFeO2 have displayed strong absorption edges determined in the near infrared region ~ 1.2 eV [16,17,18,19,20,21]; absorption in the mid-visible region ~ 2.0 eV [22,23,24,25,26]; and absorption in higher energy, from blue to ultraviolet region ~ 3.0 eV [27,28,29,30,31,32,33,34]. Several groups have also reported multi-band gap results [22, 23, 25, 26].
In terms of more complex delafossite oxides, there are reports which describe the growth and characterization of B-site substitution in the oxide such as Cr for Fe in CuFe1−xCrxO2 [34]; substitution of Ni for Cr in CuCr1−xNixO2 [35]; substitution of Mg for Cr in CuCr1−xMgxO2 [36]; and substitution of Fe for Ga in CuGa1−xFexO2 [37]. All these substitutions displaced the energy of the band gaps.
In the last case [37], the substitution of 15% Fe in CuGaO2 results in a band gap shifting from 2.55 eV down to 1.5 eV in the case of CuGa0.85Fe0.15O2. A second band gap measured at 3.75 eV in CuGaO2 remained in CuGa0.85Fe0.15O2. In spite that one band gap was shifted for the substitutional alloy, the photochemical reduction of CO2 to CO and CH4 is active at wavelengths of 320 nm (3.8 eV) in both cases showing that the band gap at 1.5 eV was not relevant for this reaction.
In terms of photo-catalytic experiments there are recent reports of successful photo-catalytic water-splitting using CuGaO2 electrodes. The CuGaO2 material has shown favorable attributes in terms of its ability to catalyze overall water-splitting of adsorbed H2O, meaning that its band edges are likely to be located at suitable energies in order to satisfy the conditions for H2O redox potential. However, Lee et al. [38] also describe reduced photocatalytic activity below UV wavelengths (hν < 3.2 eV), in spite of the fact that CuGaO2 having also shown weaker absorption edges around 2.7 eV in separate studies [37].
From this standpoint and with respect to the development of CuFeO2—based delafossite materials for water dissociation electrodes, it is desirable to determine the active band gap for efficient photo-catalysis in multiple band gap materials. This requires developing new testing techniques in addition to optical techniques which are the most common used in the literature.
In the present work we measured the conductivity of a CuFeO2 thin film when exposed to light as a function of wavelength to correlate conductivity with the optical characteristics of the sample. Since the catalytic activity of the electrode material depends on the number of holes generated by the light in the semiconductor and the number of holes able to arrive to the surface of the semiconductor (electrode), knowledge of photoconductivity properties of the material is crucial. We measured the film conductivity using van der Pauw four-contact method while illuminated with a narrow light wavelength which is obtained from a halogen lamp passing through a monochromator. The photoconductivity method is validated using a CdS film with a well determined band gap.
The high quality epitaxial nature of our CuFeO2 thin film samples was previously confirmed by TEM, XRD and Raman spectroscopy.
2 Experimental methods
2.1 Sample description
CuFeO2 (001) films were deposited onto Al2O3 (001) substrates from a stoichiometric polycrystalline CuFeO2 target using pulsed laser deposition. Details of the experimental setup and procedure have been described elsewhere [21]. The film was grown in a vacuum system at 600 °C under an O2 partial pressure of 1.33 × 10−2 Pa.
CdS films, 100 nm thick were sputtered deposited over a 1″ × 1″ quartz substrate and they were obtained from Matsurf Technologies Inc., St. Paul, Minnesota.
2.2 Sample characterization
2.2.1 Atomic force microscopy (AFM)
Atomic Force Microscopy (AFM) micrographs were measured using a commercial AFM NanoWizard 3 from JPK instruments. Scans of 10 × 10 μm and 1 × 1 μm were performed using taping mode in different areas of the samples.
2.2.2 X-ray diffraction (XRD)
A four-axis Rigaku XRD system with a Cu rotating anode (CuKα radiation) and Huber goniometers was used for determining the crystal structure of the deposited films. CuFeO2 and CuFe2O4 phases were identified using powder diffraction files no. 01-075-2146 and no. 00-025-0283, respectively.
2.2.3 Raman spectroscopy
Raman spectra were obtained using a LabRam010 system from Instruments S.A. (Horiba), featuring a 5.5 mW He–Ne laser (632.8 nm wavelength). This instrument used an Olympus confocal optical microscope with a light spectrometer in a back-scattering geometry where the incident beam was linearly polarized and spectral detection was non-polarized. The spectra were taken at room-temperature using a × 100 objective (~ 10 μm spot size) with an energy resolution of approximately 1 cm−1.
2.3 Optical measurements
A visible/NIR transmittance and reflectance spectroscopy system was set up to measure the optical response of the samples. The apparatus previously described [23] uses a 9 W tungsten-halogen model SL201 from Thorlabs. The light sources provide a broadband spectrum. The white light goes through a monochromator (Triax 180, Jobin–Yvon–Horiba) equipped with a 1200 g/mm blazed ruled grating and set up to provide near monochromatic light within a 1 nm wavelength resolution. Silicon UV enhanced photodetector are used to collect transmitted and reflected light (angle of transmittance is recorded at normal incidence (θ = 0°) while angle of reflectance is recorded at θ < 10°). The white light beam is chopped (730 Hz) before entering the monochromator and the signals are measured using lock-in amplifiers. All data is normalized to the halogen lamp spectrum.
2.4 Electrical conductivity measurements
Four metallic contacts were placed on the film. To make sure that the contacts adhered well to the surface of the CuFeO2 film a 5 nm Pt layer was e-beam evaporated followed by a 30 nm Au layer deposited via physical vapor deposition. Then, silver paint was used to attach four thin cables on top of Au contacts. The CuFeO2 film was placed in a manipulator of a high vacuum system and the four electrical contacts spot-welded to an electrical feed-through. The sample was fixed over a Macor insulating substrate using isolated stainless steel mini clamps. The sample temperature was measured with K-type thermocouple using a Fluke multimeter. The sample inside the vacuum system was facing a quartz window transparent to UV/visible/NIR light.
The electrical resistance of the sample is determined with van der Pauw [41] technique (four-point method). The current and voltages are obtained and measured with Radiant RT66-B (Ferrodevices) modified since our sample is passive and not active such as a ferroelectric film. A schematic configuration of the circuit is shown in Fig. 1. The van der Pauw method requires exchange positions between the two contacts where voltage is applied and the two contacts for sensing the current. Our results were obtained with 4 configurations and the respective reciprocal connections did not change more than 0.01%, indicating high quality electrical contacts made with the silver paint.
The monochromator used in the optical measurements equipped with the same 1200 g/mm blazed ruled grating and a broadband light from a Thorlabs SL201 (VIS-IR) is used to shine light over the CuFeO2 film while measuring its conductivity. The light wavelength is selected when passing through a wide adjustable slit in inverse configuration (Edmund Optics) which provides a narrow wavelength resolution (~ 2 mm). The light spot is then deviated by an optical mirror at 45° and focused onto the CuFeO2 thin film sample through viewport of the high-vacuum chamber using a condenser lens (focal length F = 125 mm./50.8 mm diameter—Thorlabs).
Electrical resistance measurements were made after the sample was exposed to a fixed wavelength for 20 min, taking 8 min to collect the data. Care was taken to eliminate any source of external light while the selected wavelength entered the vacuum chamber through the quartz window. Electrical resistance measurements were made at ~ 25° C and total pressure of 1 × 10−6 Torr inside the vacuum chamber. The experimental setting combining light illumination and electrical conductivity measurement is shown in Fig. 2
3 Results
3.1 Structural and morphological characterization of CuFeO2 film
An AFM image of the surfaces of the CuFeO2 film (100 nm thickness) with scanning area of 10 × 10 μm is shown in Fig. 3a and scanning an area of 2 × 2 μm is shown in Fig. 3b. The surface of the samples appears very flat at 10 × 10 μm scale. Images obtained in areas of 2 × 2 μm show an array of agglomerated with sizes less than 10 nm in diameter.
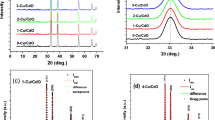
XRD and Raman characterization and analyses of the CuFeO2 films are described in detail elsewhere [21]. A summary of the results is: XRD spectra for CuFeO2 film displayed only peaks corresponding to c-axis reflections and corresponding to Miller indexes (003), (006), (009) and (0012). A typical XRD spectrum of CuFeO2 is shown in Fig. 4a. These oriented planes show the high quality epitaxial growth of CuFeO2. Although the formation of CuFe2O4 spinel material within the thicker CuFeO2 sample with small peaks corresponding to (222) oriented plane at \(2\theta = 54.6^\circ\) and (333) oriented plane at \(2\theta = 85.7^\circ\), are visible.
a XRD diffraction patterns for both CuFeO2 samples: 21 nm thick film is shown with a blue line; 75 nm thick film is shown with a red line. The cuprospinel phase is shown with the sign (*) b Raman spectra of the same samples: blue squares corresponds to the spectrum of 21 nm CuFeO2 film, red circles corresponds to the spectrum of 75 nm CuFeO2 film. Raman peaks labeled with (*) correspond to the Al2O3 substrate
Raman spectra were taken at room temperature using a 100 × objective (~ 10 μm spot size) with an energy resolution of approximately 1 cm−1. The CuFeO2 film showed a typical delafossite Raman spectrum shown in Fig. 4b with three main peaks at 350, 511 and 689 cm−1. The peak located at 350 cm−1 was attributed to the Eg vibrational mode along the c-axis of the octahedral structure; the peak at 689 cm−1 was attributed to the A1g vibrational mode along a plane perpendicular to the c-axis; the peak at 511 cm−1 was attributed to non-zero wave vector phonons typical of crystalline defects [39, 40].
3.2 Optical measurements
Transmittance spectra were recorded for a pure quartz substrate and a 100 nm CdS thin film on quartz substrate sample. The transmission spectra are displayed in Fig. 5a. Transmittance spectra were also recorded for the 75 nm CuFeO2 film and displayed in Fig. 5b. The sample is very transparent and the transmission spectrum is amplified 35 times (35×). In the same plot we show the spectra of the halogen lamp and the light through a dydimium glass use to calibrate the monochromater using the typical absorption lines of didymium.
a Plot of the transmittance spectra as a function of photon energy for the CdS sample: black line correspond to the spectrum of pure quartz substrate, red line corresponds to the spectrum of sample of 100 nm CdS film on quartz substrate. b Plot of the transmittance spectra × 35 as a function of photon energy for the CuFeO2 film. In the same plot it is shown the spectra of the halogen lamp and the spectra through a didymium glass filter used to calibrate the monochromator
Tauc-gap analyses were performed on each spectrum in order to further relate spectral absorption edges with estimations of direct and indirect band to band transition energies and the band gap from which they emerged. The transmittance spectra results were then treated in order to determine the band gap energy (Eg) of each sample using Tauc`s expression [42,43,44],
In Eq. (2), h is Planck’s constant, ν is the frequency of the incident light, α is the absorption coefficient, b is a proportional constant, and Eg is the band gap energy defined as the difference between the lowest energy level in conduction band and the highest energy level in the valence band. When linearity was identified within the Tauc plot, extrapolation to the x-axis revealed possible optical band gap energies for the sample. The plotting is repeated, once using power n = ½ so as to identify direct gap behavior and secondly using power n = 2 to identify characteristics of indirect energy gaps within the samples. For the CdS sample a Tauc plot with n = 1/2 was made and displayed in Fig. 6a. A band gap of 2.4 eV can be estimated from the intersection of the linear part of the plot with the energy scale at zero ordinate. This value is in perfect agreement with the well accepted value for CdS bulk semiconductor [46]. The Tauc plot for the 75 nm CuFeO2 film is displayed in Fig. 6b. Linear regions extrapolating at zero ordinate indicate two direct transition band gaps: 1.2 and 2.2 eV. Very few data points are in the UV region in order to ascertain a third gap at 3.0 eV.
Transmittance spectra were also recorded for a 21 nm CuFeO2 film (shown in Fig. 6b) and reported previously [23]. Both samples showed relatively stronger absorption in the NIR-(Red) region (in the range 1.0 eV < hν < 1.5 eV) and in the Vis region (1.8 eV < hν < 2.9 eV). The change in transmission is consistent with the variation in thickness and optical path of absorption.
The results for recorded energy gaps (pertaining to measurable absorption edges) for the two CuFeO2 samples from both the transmittance and reflectance measurements are summarized in Table 1.
From transmittance measurements, the first fundamental Tauc-gap was measured at 1.3 eV for the range between 1.0 and 2.0 eV, is in fair agreement with those reported in Refs. [16,17,18,19,20,21]. At higher energies, a secondary Tauc-gap was observed at 2.2 eV (in agreement with [22,23,24,25,26]) and possibly a third absorption edge pertaining to ~ 3 eV.
With regard to the small amount of cuprospinel formed on the 75 nm CuFeO2 film, Nawle et al. [47] reported a direct band gap of 1.95 eV for cuprospinel (CuFe2O4). The amount of cuprospinel in the 75 nm CuFeO2 film is very small and it does not show up in the 21 nm CuFeO2 film, Thus, cuprospinel does not affect the direct band gap of 2.2 eV measured in the CuFeO2 films. Neither affects photoconductivity of the samples.
3.3 Conductivity measurements
3.3.1 CdS conductivity
Before electrical resistance measurements as a function of wavelength were made, two critical parameters were measured under the same conditions of vacuum and temperature. First, the electrical resistance of the sample was measured in complete darkness to obtain the highest resistance value, larger than 40 kΩ. A second value of the sample resistance was obtained under illumination broadband light using the zeroth order diffraction of the monochromator (20 kΩ). This means the white light travels along the same optical path of a selected given wavelength. Subsequently, electrical resistance was measured as a function of an incoming wavelength. The electrical resistance was measured at a given wavelength within the range 460–580 nm and light wavelength was change in discrete steps of 30 nm. The resistance of the film was decreased by a factor of two right at wavelength value identical to the band gap energy (2.4 eV) of CdS as displayed in Fig. 7a. The relative small change can be explained by the small light intensity in that range of wavelength. The phenomenon known as photoconductivity resulting in increased conductivity in a sample is not very pronounced at high temperatures except when the illumination is by an intense beam of light. At low temperatures, illumination results in excitation of localized carriers to conduction or valence band [48].
3.3.2 CuFeO2 conductivity
The electrical resistance of the sample was measured in complete darkness to obtain the highest resistance value and a second value of the sample resistance was obtained under illumination broadband light using the zeroth order diffraction of the monochromator.
These values are summarized in Table 2.
The resistance difference appears insignificant but the value is consistent with the change in carriers observed in the same photoconductor (CuFeO2 sol–gel made) [49] at 0.5 sun illumination (\(0.5 \times 10^{9} \;{\text{cm}}^{ - 2} \;{\text{pulse}}^{ - 1}\)). This can be explained by the results obtained for CdS and shown in Fig. 7a. The decrease of resistivity happened right at the photon energy corresponding to the band gap energy of the semiconductor. The excess of energy of photon with larger energy than that cooresponding to the band gap is dissipated in heat, in other words, wasted.
Subsequently, electrical resistance was measured as a function of an incoming wavelength. The electrical resistance was measured at a given wavelength within the range 450–1100 nm and light wavelength was change in discrete steps with an uncertainty of \(\pm \;5\; {\text{nm}}\).
These results are displayed in Fig. 7b. All the data obtained are of a lower value than the resistance obtained in darkness. The resistance obtained with white light (zero order) illumination is close to an average value. Local minimum related to the band gaps are obtained by fitting and average line (red) due to the high scatter in the data. The red line shows gap-1 at ~ 1.4 e V (886 nm), gap-2 at ~ 2.1 eV (591 nm) and gap-3 at ~ 2.5 eV (496 nm).
The resistance data should be normalized to light intensity at different wavelengths and corrected according the halogen lamp characteristics. The halogen lamp SL201L spectrum, intensity as a function of light energy, provides by Thorlabs is displayed in Fig. 8. The intensity of the lamp is large in the IR region a small in the UV region.
The light intensity was measured using a Si photodiode (with area A = 0.3 cm2) placed between condenser lens (L3 in Fig. 2) and the quartz window of the vacuum chamber. The light intensity increases from 67 nW/cm2 for 450 nm light up to 333 nW/cm2 for 1100 nm light.
We calculated the change in resistance from the resistance measured in dark (R0) using the following equation
The change in resistance was normalized by relative light intensity I(λ), the fractional intensity at a given wavelength. A plot of \(\left( {\frac{\Delta R}{{R_{0} }}} \right)\big/A \times I\left( \lambda \right)\) as a function of photon energy of the incoming light is shown in Fig. 9. The most significant change in resistance is a rapid lineal increased which intersect the energy scale at gap-1 ~ 1.5 eV, gap-2 ~ 2.1 eV and gap-3 ~ 2.5 eV. These data rapid increase in this type of plots is related to increase in conductivity of the sample. Thus, the interpretation of Fig. 8 is that each time that the energy of the incoming light exceeds the energy gap of the semiconductor, the conductivity in the film increases. The bandgaps at 2.1 eV and 1.5 eV are in agreement with the value obtained from transmittance Tauc plots. But now we can ascertain the existence of a third gap at 2.5 eV which is in the near UV region. This experimental method of combining resistivity with radiation illumination in order to determine band gap of photo-conductors was validated using a thin film of CdS in which the fast increase in conductivity was determined at 2.40 ± 0.05 eV.
Light intensity-normalized change of resistance \({{\left( {\frac{{R(\lambda ) - R_{0} }}{{R_{0} }}} \right)} \mathord{\left/ {\vphantom {{\left( {\frac{{R(\lambda ) - R_{0} }}{{R_{0} }}} \right)} {A \times I(\lambda )}}} \right. \kern-0pt} {A \times I(\lambda )}}\) 75 nm CuFeO2 film as a function of photon energy. R0 is the resistance of the film in the dark and the units of light intensity-normalized change of resistance are 103 W−1
The absorption edges that we measured in the region of 1.0–1.3 eV can be compared and attributed to the expected 1.2–1.4 eV indirect L—Γ transitions in Ref. [24], with an expected Urbach tail formed from sub-gap indirect d–d state transitions. Both CuFeO2 samples displayed the activation of a direct transition in the 2.0–2.2 eV region. This absorption edge can be attributed to the activation of direct L-point transitions and direct Γ-point transitions calculated to be at 1.6 eV and 2.4 eV respectively [24]. It is possible that the absorption observed in this spectral region takes place by a super positioning of direct transitions over both symmetry points. Each spectrum obtained also indicates the activation of a possible higher absorption edge located in the range hν > 3.1 eV. These effects are particularly prominent in the reflectance spectra [26] and such wide band-energy gaps have been reported before [22]. These absorption edges are likely to result from valence band to second conduction band transitions or to absorption via channels located higher up within the conduction bands which are known to possess relatively large optical cross-sections. These higher energy absorption channels are thought to form an apparent optical edge. This explanation is explored in detail by Nie et al. [45].
4 Conclusions
The photoconductivity of a well characterized epitaxial thin film of CuFeO2 was measured as a function of near monochromatic light selected from a W-halogen lamp through a monochromator. The film conductivity was obtained using van der Pauw four-contact method while illuminating the film. The conductivity shows mark jumps between 1.5 eV, 2.1 and 2.5 eV which are well correlated with the optical absorption results. Analysis of Tauc plot from optical data for the CuFeO2 thin films showed predominant absorption edges at 1.2 ± 0.2 eV and 2.2 ± 0.2 eV. The higher energy gap at ~ 2.5 eV seeing in resistance measurements was not clearly determined from optical transmittance due to the poor response of the Si diodes at UV wavelengths. The photoconductivity technique implemented to determined band-gaps for CuFeO2 was validated by doing same measurement in a CdS film for which a bad gap of 2.4 eV was observed. Further investigations of the carrier mobility and photo-catalytic reactions on the sample surfaces will help determine the impact of the substituted layers and surface absorption channels on the generation of the electron–hole pairs required for photo-catalytic processes within the materials investigated.
References
Reasons for increase in demand for energy, BBC online (2019) https://www.bbc.com/bitesize/guides/zpmmmp3/revision/1. Accessed 20 Nov 2018
Angelis-Dimakisa A, Biberacherb M, Dominguez J, Fioresed G, Gadochab S, Gnansounouf E, Guarisod G, Kartalidisa A, Panichellif L, Pinedoc I, Robbag M (2011) Methods and tools to evaluate the availability of renewable energy sources. Renew Sustain Energy Rev 15(2):1182–1200
Andrews John, Shabani Bahman (2012) Re-envisioning the role of hydrogen in a sustainable energy economy. Int J Hydrog Energy 37:1184
Dresselhaus MS, Thomas IL (2011) Alternative energy technologies. Nature 414:332–337
Lao SJ, Qin HY, Ye LQ, Liu BH, Li ZP (2010) A development of direct hydrazine/hydrogen peroxide fuel cell. J Power Sources 195(13):4135–4138
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238(5385):37–38
Finlayson AP, Tsaneva VN, Lyons L, Clark M, Glowacki BA (2016) Evaluation of Bi-W-oxides for visible light photocatalysis. Phys Status Solidi A 203(2):327–335
Gratzel Michael (2001) Photoelectrochemical cells. Nature 414:338–344
Maeda K (2011) Photocatalytic water splitting using semiconductor particles: history and recent developments. J Photochem Photobiol C: Photochem Rev 12(4):237–268
Shockley W, Quiesser HJ (1961) Detailed balance limit of efficiency of p–n junction solar cells. J Appl Phys 32(3):510–519
Sivula K, Van de Krol R (2016) Semiconducting materials for photoelectrochemical energy conversion. Nat Rev Mater 2016:15010
Abdi FF, Berglund S (2017) Recent development in complex metal oxide photoelectrodes. J Phys D Appl Phys 50:193002
Huda MN, Yan Y, Walsh A, Wei S-H, Al-Jassim MM (2009) Group-IIIA versus IIIB delafossites: electronic structure study. Phys Rev B 80:035205
Galakhov VR, Poteryaev AI, Kurmaev EZ, Anisimov VI, Bartkowski St, Neumann M, Lu ZW, Klein BM, Zhao Tong-Rong (1997) Valence-band spectra and electronic structure of CuFeO2. Phys Rev B 56:4584–4591
Hiraga H, Makino T, Fukumura T, Weng H, Kawasaki M (2011) Electronic structure of the delafossite-type CuMO2 (M = Sc, Cr, Mn, Fe, and Co): optical absorption measurements and first-principles calculations. Phys Rev B 84:041411(R)
Tablero Crespo C (2018) Potentiality of CuFeO2-delafossite as a solar energy converter. Sol Energy 163:162–166
Pavunny S, Kumar A, Katiyar RS (2010) Raman spectroscopy and field emission characterization of delafossite CuFeO2. J Appl Phys 107:013522
Omeiri et al (2009) Electrochemical and photoelectrochemical characterization of CuFeO2 single crystal. J Solid State Electrochem 13:1395–1401
Barot N et al (2017) Structure, I–V characteristics and optical studies of delafossite CuFeO2 and CuFe0.96Ti0.03V0.01O2 prepared under high vacuum. In: AIP conference proceedings, vol 1837, p 040031
Read Carrie G, Park Y, Choi K (2012) Electrochemical synthesis of p-type CuFeO2 electrodes for use in a photoelectrochemical cell. J Phys Chem Lett 3:1872–1876
Joshi Toyanath, Senty Tess R, Trappen Robbyn, Zhou Jinling, Chen Song, Ferrari Piero, Borisov Pavel, Song Xueyan, Holcomb Mikel B, Bristow Alan D, Cabrera Alejandro L, Lederman David (2015) Structural and magnetic properties of epitaxial delafossite CuFeO2 thin films grown by pulsed laser deposition. J Appl Phys 117:013908
Benko FA, Koffyberg FP (1987) Opto-electronic properties of p- and n-type delafossite, CuFeO2. J Phys Chem Solids 48:431–434
Wheatley RA et al (2017) Comparative study of the structural and optical properties of epitaxial CuFeO2 and CuFe1−xGaxO2 delafossite thin films frown by pulsed laser deposition methods. Thin Solid Films 626:110–116
Khuong Ong K, Bai P Blaha, Wu P (2017) Electronic structure and optical properties of AFeO2 (A = Ag, Cu) within GGA calculations. Chem Mater 19:634–640
Riveros G, Garín C, Ramírez D, Dalchiele EA, Marotti RE, Pereyra CJ, Spera E, Gómez H, Grez P, Martín F, Ramos-Barrado JR (2015) Delafossite CuFeO2 thin films electrochemically grown from a DMSO based solution. Electrochim Acta 164:297–306
Prevot Mathieu S, Jeanbourquin Xavier A, Bourée Wiktor S, Abdi Fatwa, Friedrich Dennis, van de Krol Roel, Guijarro Néstor, Le Formal Florian, Sivula Kevin (2015) Enhancing the performance of a robust sol–gel-processed p-type Delafossite CuFeO2 photocathode for solar water reduction. Chemsuschem 8:1359–1367
Zhu T, Deng Z, Fang X, Dong W, Shao J, Tao R, Wang S (2016) Room temperature deposition of amorphous p-type CuFeO2 and fabrication of CuFeO2/n-Si heterojunction by RF sputtering method. Bull Mater Sci 39(3):883–887
Bera A, Deb K, Sinthika S, Thapa R, Saha B (2018) Chemical modulation of valence band in delafossite structured CuFeO2 thin film and its photoresponse. Mater Res Express 5:015909
Chen Hong-Ying, Fu Guan-Wei (2014) Influences of post-annealing conditions on the formation of delafossite-CuFeO2 thin films. Appl Surf Sci 288:258–264
Deng Z, Fang X, Wang X, Wu S, Dong W, Shao J, Tao R (2015) Characterization of amorphous p-type transparent CuFeO2 thin films prepared by radio frequency magnetron sputtering method at room temperature. Thin Solid Films 589:17–21
Abdelwahab HM, Ratep A, Abo Elsoud M, Boshta M, Osman MBS (2018) Influences of annealing temperature on sprayed CuFeO2 thin films. Results Phys 9:1112–1115
Chen H-Y, Wu J-H (2012) Characterization and optoelectronic properties of sol–gel-derived CuFeO2 thin films. Thin Solid Films 520:5029–5035
Alkhayatt AHO, Thahab SM, Zgair IA (2016) Structure, surface morphology and optical properties of post-annealed delafossite CuFeO2 thin films. Optik 127:3745–3749
Lalanne M, Barnabe A, Mathieu F, Tailhades Ph (2019) Synthesis and thermostructural studies of a CuFe1−xCrxO2 delafossite solid solution with 0 ≤ x ≤ 1. Inorg Chem 48:6065–6071
Zheng SY, Jiang GS, Su JR, Zhu CF (2006) The structural and electrical property of CuCr1−xNixO2 delafossite compounds. Mater Lett 60:3871–3873
Lim SH, Desu S, Rastogi AC (2008) Chemical spray pyrolysis deposition and characterization of p-type CuCr1−xMgxO2 transparent oxide semiconductor thin films. J Phys Chem Solids 69:2047–2056
Lekse JW, Underwood MK, Lewis JP, Matranga C (2012) Synthesis, characterization, electronic structure, and photocatalytic behavior of CuGaO2 and CuGa1–xFexO2 (x = 0.05, 0.10, 0.15, 0.20) delafossites. J Phys Chem C 116:1865–1872
Lee M, Kim D, Yoon YT, Kim YI (2014) Photoelectrochemical water splitting on a delafossite CuGaO2 semiconductor electrode. Bull Korean Chem Soc 35(11):3261–3266
Salke NP, Kamali K, Ravindran TR, Balakrishnan G, Rao R (2015) Raman spectroscopic studies of CuFeO2 at high pressures. Vib Spectrosc 81:112–118
Aktas O, Truong KD, Otani T, Balakrishnan G, Clouter MJ, Kimura T, Quirion G (2012) Raman scattering study of delafossite magnetoelectric multiferroic compounds: CuFeO2 and CuCrO2. J Phys: Condens Matter 24:036003
van der Pauw LJ (1958) A method of measuring the resistivity and hall coefficient of lamellae of arbitrary shape. Philips Res Rept 13:1–9
Tauc J (1968) Optical properties and electronic structure of amorphous Ge and Si. Mater Res Bull 3:37–46
Pankove JI (1971) Optical processes in semiconductors. Prentice Hall, Upper Saddle River
Tauc J (1972) In: Abeles F (ed) Optical properties of solids. North-Holland, Amsterdam, p 227
Nie X, Wei S, Zhang SB (2002) Bipolar doping and band-gap anomalies in delafossite transparent conductive oxides. Phys Rev Lett 88:066405
Collaboration: Authors and editors of the volumes III/17B-22A-41B (1999) Cadmium sulfide (CdS) band structure, energy gap, cubic modification. In: Madelung O, Rössler U, Schulz M (eds) II-VI and I-VII compounds; semimagnetic compounds. Landolt-Börnstein—Group III Condensed Matter (Numerical Data and Functional Relationships in Science and Technology), vol 41B. Springer, Berlin
Nawle Anant C, Humbe Ashok V, Babrekar MK, Deshmukh SS, Jadhav KM (2017) Deposition, characterization, magnetic and optical properties of Zn doped CuFe2O4 thin films. J Alloys Compd 695:1573–1582
(2009) Physics of semiconductor devices. Lecture 35: photoconductivity, NPTEL. A project funded by MHRD, Govt. of India https://nptel.ac.in/about.php. Accessed Nov 2018
Prevot Mathieu S, Jeanbourquin Xavier A, Bouree Wiktor S, Abdi Fatwa, Friedrich Dennis, van de Krol Roel, Guijarro Nestor, Le Formal Florian, Sivula Kevin (2017) Evaluating charge carrier transport and surface states in CuFeO2 photocathodes. Chem Mater 29:4952–4962
Acknowledgements
Thanks are due to Dr. T. Joshi and Professor D. Lederman (UC, Santa Cruz) for providing the samples of CuFeO2 films, grew by PLD at West Virginia University. This work was supported by FONDECyT Proyecto Anillo ACT1409 (Comisión Nacional de Investigación Científica y Tecnológica).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vojkovic, S., Fernandez, J., Elgueta, S. et al. Band gap determination in multi-band-gap CuFeO2 delafossite epitaxial thin film by photoconductivity. SN Appl. Sci. 1, 1322 (2019). https://doi.org/10.1007/s42452-019-1387-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1387-2