Abstract
An attempt was made to optimize the key parameters of acid catalyst esterification and base catalyst transesterification processes for higher biodiesel yield from mahua oil with butanol using traditional optimization procedure. In esterification stage quantity of acid catalyst and molar ratio of butanol to mahua oil were considered for optimization of lowest value of free fatty acid (FFA). In transesterification stage molar ratio of butanol to mahua oil, quantity of alkaline catalyst, process temperature, process time and stirring speed were considered for maximum yield of mahua oil butyl ester. From the results, 6:1 molar ratio of butanol to mahua oil, 2% (w/w) concentrated sulphuric acid (H2SO4) were identified as optimized values with 1.1% FFA value which was reduced from 19.8%. In the second stage results, 6:1 molar ratio of butanol to oil, 1.5% (w/w) potassium hydroxide (KOH), 80 °C process temperature, 90 min process time and 500 rpm stirring speed were recorded as optimized values with 94.8% yield of mahua oil butyl ester. The butyl ester produced with optimum values of transesterification process parameters has been characterized and ensured the satisfaction of EN14214 biodiesel standards requirements.
Graphic abstract

Similar content being viewed by others
1 Introduction
Increased population, globalization, industrial revolution are the major reasons for increased power consumption and transportation in developing countries such as India. Due to high power consumption and increased transportation, two major threats faced by the globe for the past few decades are fossil fuel depletion and production of dangerous pollutants from fossil fuel combustion [1,2,3]. Hence, identification of alternative to fossil fuel, particularly to diesel has become most important nowadays. Biodiesel is such an auxiliary resource for fossil diesel in diesel engine applications. Mono alkyl esters of any fatty acid from triglycerides of vegetable oils or animal fats are known as biodiesel. Transesterification is one of the economic, robust, fast and simple chemical processes to produce biodiesel from triglyceride [4,5,6,7].
Characteristics and amount of alcohol and catalyst, process time and temperature, stirring or agitation speed, and moisture content in the raw materials are major parameters affecting the quality and amount of yield of biodiesel [8, 9]. Out of these parameters, the class of alcohol used affects the amount of yield and quality of biodiesel. The optimum level of all other parameters are also significantly affected by the class of alcohol used. For example, for the same oil and same catalyst with different alcohols will have different levels of other parameters for maximum yield of biodiesel [10, 11]. Methanol is widely used alcohol to produce biodiesel and ethanol to some extent. Other higher molecular weight alcohols like pentanol, propanol and butanol are very rarely utilized for biodiesel synthesis. Among them butanol has some interesting advantages such has higher miscibility with lipid resources, higher contribution to initial mass transfer in reaction, higher boiling point of butanol permits conduction of reaction process at higher temperature to achieve faster rate of reaction [12,13,14,15].
The value of acid number or quantity of free FFA content of raw oil is one of the key parameters to decide the suitable transesterification process. Higher FFA content of raw oil (2.5% and above) requires pre-treatment prior to transesterification process. In pre-treatment step esterification process is carried out with acid catalyst to reduce the acid value to required amount. If the value of FFA is not reduced less than 2% in the first step, further esterification also can be repeated. Once the amount of FFA is reduced considerably, transesterification process will be carried with base catalyst [7, 16,17,18,19,20,21,22,23].
The quality of the biodiesel is drastically affected by total count of carbon in the structure, amount of carbon double bond in the structure and quantity of unsaturated fatty acid of the resource oil. The relationship between physicochemical properties of biodiesel and chemical composition of the raw oil is very important to select the desirable resource for good quality biodiesel [24,25,26,27,28,29,30]. The characteristics of flow of the biodiesel at low temperatures are improved when the raw oil contains low level of saturated fatty acids. Raw oil with low unsaturated fatty acid group will improve the oxidation stability and cetane number of the biodiesel. The viscosity and density are two controversial properties. The degree of saturation and chain length of the raw oil are directly proportional to the viscosity and inversely proportional to the density. At the outset raw oils with high mono unsaturated and low in both saturated and poly unsaturated fatty acid will produce good quality biodiesel [31,32,33,34,35,36,37,38].
Biodiesel feedstock supply chain in India facing two major illnesses such as lack of cultivation and seed processing. The government policy on the utilization of waste land for cultivation of biodiesel feedstocks like Jatropha and other tree born feed stocks and investment policy of private sectors for cultivation should be jointly derived to overcome the above mentioned illnesses. The high quality planting seeds, latest cultivation technology, increased marketing opportunity, subsidy for cultivation and guaranteed profit for the formers are some strategies to be implemented for increase the supply chain of biodiesel feedstock.
The first generation biodiesel feedstocks are edible vegetable oils. Due to the increased population, the demand for edible crops has been increased many folds. Hence, the utilization of edible feed stocks for energy production is not encouraged. At the same time, the increase in greenhouse gas emission in the globe has been increased to the alarming level due to fossil fuel combustion. This overshooting of CO2 level in the earth should be suppressed by replacing fossil fuel by renewable fuel. Hence, it is mandatory to produce non-edible feedstocks for bioenergy production and also it is important to share the available land for cultivating food products, livestock production and crops for bioenergy production equally.
Mahua (Madhuca indica) which belongs to sapotaceae family is tree born seed oil available widely in north and southern parts of India. From the literature it is found that mahua oil is rich in mono unsaturated and poor in both saturated and polyunsaturated fatty acid group, which is well proved best suitable to biodiesel production. But no literature is contributed for butyl ester production from mahua oil. Hence, an attempt was made to optimize the butyl ester production from mahua oil and this research article is reporting the results of optimization of esterification and transesterification process parameters of butyl ester production from mahua oil.
2 Materials and method
2.1 Materials and experimental setup
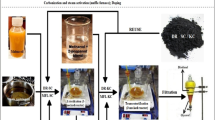
The well ripened mahua seeds were collected from the local villages in and around Chennai, India. 98% pure concentrated sulphuric acid, pellet form laboratory grade potassium hydroxide with 85% purity and 99% pure butanol were used in this experimental work. The experimental setup consists of a 500 ml capacity borosilicate glass reactor with three necks, speed governable mechanical stirrer with Remi stirrer motor carrying glass shaft and Teflon blades, heating mantle with temperature regulator and a thermometer of 0–100 °C range. The schematic diagram of the experimental setup is shown in Fig. 1.
2.2 Oil extraction and biodiesel preparation procedure
The collected mahua seeds were dried in the sunlight for about 48 h and the shells were removed manually. Then the dried kernels were crushed in a mechanical oil crusher and raw oil was collected. The collected oil was filtered using fine mesh cloth filter for removing the solid contaminants and heated in a beaker up to nearer the boiling point to evaporate the water content present in the oil and cooled down to room temperature. The flow chart showing the steps involved in mahua oil butyl ester production is given in Fig. 2.
In the esterification process, two key process parameters such as butanol to oil molar ratio and quantity of acid catalyst were taken for optimization and other parameters like process temperature, process time and stirring speed were kept constant as 60 °C, 1 h and 500 rpm respectively. 100 (± 0.1) grams of pre-treated oil was placed in the reactor and gradually heated up to the necessary temperature under the fixed stirring speed. Meanwhile the essential amount of butanol and concentrated H2SO4 were exactly measured and mixed thoroughly and added to the oil in the reactor once the required temperature was attained. This time was noted as the commencement time of the reaction. Once the decided time was attained the reactants were transferred to settling beaker and settled for 12 h. The top layer of butanol and water emulsion was removed and the bottom esterified product was used for testing the FFA value. Until the FFA value is reduced less than 2%, the process parameters were optimized.
In Transesterification process, five foremost process parameters such as molar ratio of butanol to mahua oil, quantity of KOH, process temperature, process time and stirring speed were considered for optimization. 100 (± 0.1) grams of esterified product was taken in the reactor and heated up to the necessary temperature under the prefixed stirring speed. The required quantity of butanol and KOH pellets were exactly measured and mixed thoroughly and added to the oil in the reactor once the required temperature was attained. This time was noted as the commencement time of the transesterification reaction. Once the decided time was attained the reactants were transferred to settling beaker and undisturbed for 24 h.
The optimal value of a particular parameter in the esterification and transesterification process was obtained by conducting a series of experiments with different values of that particular parameter and keeping the other parameters constant at any random value [13]. Once the optimal value was attained for that particular parameter, that value was fixed constant for the optimization of the other parameters [13].
2.3 Oil and biodiesel characterization
Fatty acid profile of the raw mahua oil was estimated using a Perkin-Elmer Clarus 500 Auto System XL with elite series PE-5 capillary column equipped with mass spectrometer. Kinematic viscosity was measured at 40 °C using a Brookfield DV-II Proviscometer as per the procedure of ASTM D 445. The pour point and the cloud point were simultaneously estimated in accordance with ASTM D 5949 and ASTM D 5773 respectively. Flash point was measured using Pensky Martene open cup apparatus. Heating value was determined with the use of Parr—6772 bomb calorimeter. Density at 15 °C was measured using a Rudolph DDM 2909 Automatic Density Meter. The values of iodine number and cetane number were calculated as per the standards of ASTM. The acid value was determined using a suitable titration with standardized KOH solution with phenolphthalein as the indicator [34].
3 Result and discussion
3.1 Fatty acid profile and physicochemical properties of mahua oil
The fatty acid profile and their weight content in percentage of mahua oil were presented in Table 1. From Table 1 it was found that oleic acid was the dominating fatty acid which shares 46.5% in the total weight of mahua oil. The total amount of saturated fatty acid content was 34.9%, total amount of monounsaturated fatty acid content was 46.5% and the total amount of polyunsaturated fatty acid content was 18.6%. From the above results it was perceived that mahua oil is rich in monounsaturated fatty acid, hence, suitable for high quality biodiesel production. Table 2 shows the physicochemical properties of raw mahua oil are at par with other vegetable oils. A crucial property to decide the suitability of raw oil as fuel for diesel engine is kinematic viscosity and its value for mahua oil at 40 °C was 42.32 (mm2/s). It was approximately 15 times greater than the fossil diesel, therefore raw mahua oil is not suitable for diesel engine application as fuel. Hence it is to be converted as biodiesel through two step transesterification process because the FFA content of the oil was 19.8 (% FFA as oleic acid).
3.2 Optimization of esterification process parameters
3.2.1 Effect of amount of acid catalyst
The variation of the FFA with the respective amount of acid catalyst is shown in Fig. 3. In this study the amount of H2SO4 was varied from 0.5% (w/w) to 2.5% (w/w) in the incremental step of 0.5%. While other parameters were kept constant, a 6:1 molar ratio of butanol to oil, at 60 °C, with 60 min of reaction time and 500 rpm stirring speed was used during the esterification. From the Fig. 3 it was perceived that the conversion rate of FFA to esters was greatly influenced by the variation in amount of H2SO4. It was identified that there was no appreciable reduction in FFA at 0.5% catalyst concentration and around 75% of FFA were converted into esters at 1% catalyst concentration. At 0.5% catalyst concentration the rate of conversion was very low because of inadequate amount of catalyst to speed up the process. The optimum level of acid catalyst amount was identified as 2% with corresponding optimum FFA value of 1.1% and further increase in amount of catalyst had no effect in FFA conversion, because the maximum conversion has already happened and process has been stagnated therefore no improvement in the conversion.
3.2.2 Effect of butanol to oil molar ratio
Figure 4 shows the effect of butanol to oil molar ratio on the conversion of FFA into esters. In this set of experiments the molar ratio of butanol to oil was varied from 3:1 to 7:1. The amount of acid catalyst was set to its optimal level obtained in previous sections and other parameters were set as same as in the previous section. From the Fig. 4 it was identified that the rate of reduction of FFA value was greatly affected by the amount of butanol. At the molar ratio levels 3:1 and 4:1 there were no significant reduction in FFA value, this is due to the inability of lower amount of butanol to hold the faster rate of reaction. The rate of conversion of FFA to ester at the molar ratio levels 5:1 and 6:1, was very high and the optimum level was identified as 6:1. The value of free fatty acid at molar ratio 6:1 was recorded as 1.1%. Beyond 6:1 molar ratio the further increase in amount of butanol had no effect on FFA conversion, because the higher amount of alcohol present in the esterification process will lead to water formation during reaction.
3.3 Optimization of transesterification process parameters
3.3.1 Effect of butanol to oil molar ratio on butyl ester yield
The amount of alcohol plays a major role and has greater influence on biodiesel yield in transesterification process. The stoichiometric molar ratio of alcohol to any triglyceride for transesterification is 3:1, but the transesterification process is highly reversible in nature. Therefore excess alcohol is needed to maintain the rate of forward reaction. At the same time, very high amount of alcohol in the process will emulsify the reaction due to the water formation during reaction. Hence, identification of optimum amount of alcohol is very much important. The effect of amount of butanol on butyl ester yield was shown in Fig. 5. In this study the butanol amount was varied from molar ratio 4:1 to 12:1 in the interval of 2. While other parameters were kept constant, a 1% KOH, at 60 °C, with 60 min of reaction time and 500 rpm stirring speed was used during the transesterification. It was clearly identified from the Fig. 5 that highest biodiesel yield was attained as 92.8% at 8:1 molar ratio. Since butanol is less reactive compared to methanol, higher amount of butanol was consumed in this process. If the molar ratio is increased beyond 8:1, slight drop in yield of biodiesel was noticed. This is due to the water formation of excess alcohol during the reaction which will reduce the rate of conversion. Even though 8:1 molar ratio recorded high percentage of butyl ester yield, 6:1 was considered as optimized level due to the reason that it produced result at par with 8:1.
3.3.2 Effect of catalyst amount on butyl ester yield
Figure 6 depicts the effect of variation of catalyst amount on the variation of butyl ester yield. Investigations were made by varying the catalyst concentration from 0.5 to 2.5% (w/w). The optimum value of butanol to oil molar ratio as 6:1 was set and other parameters were kept constant as same as previous section. Catalyst concentration had high influence than that of all other parameters which was evident from Fig. 6. This is due to the rate of change of biodiesel yield with respect to variation of catalyst concentration was very high. 0.5% catalyst amount was inadequate to aggravate the reaction process and hence, only 72.8% yield was produced. The maximum biodiesel yield was recorded as 93% for the catalyst concentration 1.5%. Further increase in catalyst amount had negative effect on reaction and the butyl ester yield was significantly reduced and also higher amount of soap formation was noted. This is due to the higher rate of saponification reaction of excess KOH with triglycerides.
3.3.3 Effect of process temperature on butyl ester yield
The temperature of the transesterification reaction is one of the key parameters to improve the biodiesel conversion. Higher the temperature higher the yield in general, because higher temperature leads to easy braking of cohesive bonding between triglyceride molecules and make them active in transesterification conversion. At the same time if the temperature is set greater than the boiling point of any reactant will lead to evaporation of that reactant from the process and further reduces the yield of biodiesel. The higher boiling point of butanol helps in conducting the experiments with high temperature. Figure 7 shows the variation of butyl ester yield with respect to variation of temperature. Five different temperatures were set between 50 and 90 °C with 10 °C interval. The optimal values of molar ratio and catalyst concentration obtained from previous sections were adopted and other factors were kept constant. The optimal value of biodiesel yield as 93.5% at 90 °C. At the same time the biodiesel yield for 80 °C was 93.4%. The difference between the biodiesel yield at temperature 80 °C and 90 °C is 0.1% which is very meager. The energy required for raising 10 °C temperature is very significant than the increase in the yield, hence the optimal value of temperature is considered as 80 °C.
3.3.4 Effect of process time on butyl ester yield
The rate of transesterification reaction in the beginning of the process is high. More than 50% of ester conversion will happen in first 10 to 15 min of reaction. On the other hand, the optimum yield will be attained very late due to hydrolysis reaction of oil and alcohol in later stage. Hence, the identification of optimal reaction time is very important for biodiesel yield. The amount of butyl ester yield at various process times are given in Fig. 8. Except stirring speed all other parameters were set to their optimal values and stirring speed was set to 500 rpm. The experiments were conducted at five different reactions times. The optimal value of yield of butyl ester was obtained at 90 min reaction time. Long reaction time beyond 90 min recorded lower biodiesel yield. This is due to the initiation of hydrolysis reaction by the additional residence time of reactants. The backward reaction was initiated at longer reaction times and more soap formation was also identified at the end of the reaction.
3.3.5 Effect of stirring speed on butyl ester yield
Triglyceride and alcohol are two immiscible liquids taking part in the transesterification process. Therefore the area of contact between the oil and alcohol is very limited. The area of contact between alcohol and oil will be increased by continuous stirring or agitating the reactants at optimal speed. The change in butyl ester yield with respect to the change in stirring speed is shown in Fig. 9. The stirring speed in this set of experiments was varied from 300 to 700 rpm with 100 rpm incremental step. All other parameters were set to their optimal levels. At lower speeds such as 300 rpm and 400 rpm, the yield value of biodiesel was around 80 to 85% only. This is due to the reason that at lower speeds the level of heterogeneity of the oil alcohol mixture was not reduced reasonably and the level of homogeneity of the mixture was not appreciably improved. At the same time further increase in stirring speed beyond 500 rpm had negative effect. There was reduction in yield of biodiesel at speeds greater than 500 rpm. This is due to that during the experiments with higher stirring speeds, splashing of reactants all around the reactor was noticed and hence, the improper interface between the oil and alcohol had pulled down the yield value. The results showed that the optimal value of butyl ester yield was recorded as 94.8% for 500 rpm. This is because at optimal speed of mixing the mass transfer of triglyceride from oil to interface with alcohol is appreciably increased and also the kinetics of transesterification process was altered and better results were attained.
3.4 Properties of mahua oil butyl ester
Key physicochemical properties of mahua oil butyl ester were estimated using ASTM standards and reported in Table 3. The comparison between estimated properties and EN 14214 biodiesel standards were also reported in Table 3. The major properties of the mahua oil butyl ester are comparable with other biodiesels [14,15,16]. From the results it was confirmed that the requirements of EN14214 biodiesel standards were met by all the properties of mahua oil butyl ester and hence, it can be a suitable alternate fuel to fossil diesel in diesel engine applications.
4 Conclusion
In this experimental analysis the oil extraction, fatty acid profile, physicochemical properties, and optimization of mahua oil butyl ester production where investigated and reported. The FFA value of the raw mahua oil was estimated as 19.8%, hence, two step transesterification process was adapted. In the esterification step two significant parameters such as butanol to oil molar ratio and amount of acid catalyst were optimized. In the second step, key transesterification process parameters such as molar ratio of butanol to oil, amount of alkaline catalyst, process temperature, process time and stirring speed were optimized. Based on the experimental results the following major conclusions were made.
-
Oleic acid was the dominating fatty acid which shares 46.5% in the total weight of mahua oil.
-
Mahua oil is rich in monounsaturated fatty acid hence, suitable for high quality biodiesel production.
-
6:1 molar ratio of butanol to mahua oil, 2% (w/w) concentrated H2SO4 were identified as optimized values with 1.1% FFA value which was reduced from 19.8%.
-
6:1 molar ratio of butanol to oil, 1.5% (w/w) KOH, 80 °C process temperature, 90 min process time and 500 rpm stirring speed were recorded as optimized values with 94.8% yield of mahua oil butyl ester.
-
These optimized parameters are well suitable for batch type biodiesel production industries of small and medium scale capacity and will not be suitable for continuous production.
-
The properties of the mahua oil butyl ester observed are in compliance with the EN 14214 biodiesel standard.
References
Acharya N, Nanda P, Panda S, Acharya S (2016) Analysis of properties and estimation of optimum blending ratio of blended mahua biodiesel. Eng Sci Technol Int J 20:511–517
Canakci M, Van Gerpen J (1999) Biodiesel production via acid catalysts. Trans ASAE 42(5):1203–1210
Agarwal D, Kumar L, Agarwal AK (2008) Performance evaluation of a vegetable oil fuelled compression ignition engine. Renew Energy 33:1147–1156
Atabani AE, Silitonga AS, Ong HC, Mahlia TMI, Masjuki HH, Badruddin IA, Fayaz H (2013) Non-edible vegetable oils: a critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew Sustain Energy Rev 18:211–245
Kazancev K, Sendzikiene E, Kazanceva I (2015) Application of enzymatic process for biodiesel synthesis from vegetable oil with high fatty acid content using butanol. Eng Rural Dev 20(5):302–306
Kumar D, Verma B (2018) Development of enzymatic biodiesel from vegetable oil and quantification of fatty acid butyl esters. Rasayan J Chem 11(1):187–194
Ilmi M, Abduh MY, Hommes A, Winkelman JGM, Hidayat C, Heeres HJ (2018) Process intensification of enzymatic fatty acid butyl ester synthesis using a continuous centrifugal contactor separator. Ind Eng Chem Res 57(2):470–482
Knothe G (2005) Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol 86(10):1059–1070
Singh AP, Anbumani K (2011) A comparative study on the use of butyl esters of soyabean and sunflower oils as biodiesel fuel for compression ignition engine. Iran J Mech Eng 12(1):68–85
Homan T, Shahbaz K, Farid MM (2017) Improving the production of propyl and butyl ester-based biodiesel by purification using deep eutectic solvents. Sep Purif Technol 174:570–576
Canakci M, Sanli H (2008) Biodiesel production from various feedstocks and their effects on the fuel properties. J Ind Microbiol Biotechnol 35(5):431–441
Neto BADS, Alves MB, Lapis AAM, Nachtigall FM, Eberlin MN, Dupont J, Suarez PAZ (2007) 1-n-Butyl-3-methylimidazolium tetrachloro-indate (BMI · InCl4) as a media for the synthesis of biodiesel from vegetable oils. J Catal 249(2):154–161
Eevera T, Rajendran K, Saradha S (2009) Biodiesel production process optimization and characterization to assess the suitability of the product for varied environmental conditions. Renew Energy 34(3):762–765
Sadeq MS, Demidov IN (2012) Development of technology obtaining fatty acid of butyl esters. East Eur J Adv Technol 3(57):21–24
Ghadge SV, Raheman H (2005) Biodiesel production from mahua (Madhuca indica) oil having high free fatty acids. Biomass Bioenergy 28(6):601–605
Ghadge SV, Raheman H (2006) Process optimization for biodiesel production from mahua (Madhuca indica) oil using response surface methodology. Biores Technol 97(3):379–384
Godiganur S, Suryanarayana Murthy CH, Reddy RP (2009) 6BTA 5.9 G2-1 Cummins engine performance and emission tests using methyl ester mahua (Madhuca indica) oil/diesel blends. Renew Energy 34(10):2172–2177
Hariram V, Vagesh Shangar R (2011) Characterization and optimization of biodiesel production from crude Mahua oil by two stage transesterification. Am J Eng Res 3(11):233–239
Sanli H, Canakci M (2008) Effects of different alcohol and catalyst usage on biodiesel production from different vegetable oils. Energy Fuels 22(4):2713–2719
Jena PC, Raheman H, Prasanna Kumar GV, Machavaram R (2010) Biodiesel production from mixture of mahua and simarouba oils with high free fatty acids. Biomass Bioenergy 34(8):1108–1116
Puhan S, Vedaraman N, Rambrahamam BV, Nagarajan G (2005) Mahua (Madhuca indica) seed oil: a source of renewable energy in India. J Sci Ind Res 64:890–896
Kaul S, Saxena RC, Kumar A, Negi MS, Bhatnagar AK, Goyal HB, Gupta AK (2007) Corrosion behavior of biodiesel from seed oils of Indian origin on diesel engine parts. Fuel Process Technol 88(3):303–307
Knothe G, Steidley KR (2007) Kinematic viscosity of biodiesel components (fatty acid alkyl esters) and related compounds at low temperatures. Fuel 86(16):2560–2567
Mahalingam A, Devarajan Y, Radhakrishnan S, Vellaiyan S, Nagappan B (2018) Emissions analysis on mahua oil biodiesel and higher alcohol blends in diesel engine. Alex Eng J 57(4):2627–2631
Meher LC, Dharmagadda VSS, Naik SN (2006) Optimization of alkali-catalyzed transesterification of Pongamia pinnata oil for production of biodiesel. Biores Technol 97(12):1392–1397
Nayak SK, Pattanaik BP (2014) Experimental investigation on performance and emission characteristics of a diesel engine fuelled with mahua biodiesel using additive. Energy Procedia 54:569–579
Puhan S, Vedaraman N, Sankaranarayanan G, Bharat Ram BV (2005) Performance and emission study of Mahua oil (Madhuca indica oil) ethyl ester in a 4-stroke natural aspirated direct injection diesel engine. Renew Energy 30(8):1269–1278
Puhan S, Vedaraman N, Ram BVB, Sankarnarayanan G, Jeychandran K (2005) Mahua oil (Madhuca Indica seed oil) methyl ester as biodiesel—preparation and emission characteristics. Biomass Bioenergy 28(1):87–93
Raheman H, Ghadge SV (2007) Performance of compression ignition engine with mahua (Madhuca indica) biodiesel. Fuel 86(16):2568–2573
Rajendra M, Jena PC, Raheman H (2009) Prediction of optimized pretreatment process parameters for biodiesel production using ANN and GA. Fuel 88(5):868–875
Saravanan N, Nagarajan G, Puhan S (2010) Experimental investigation on a DI diesel engine fuelled with Madhuca Indica ester and diesel blend. Biomass Bioenergy 34(6):838–843
Sabariswaran K, Selvakumar S, Kathirselvi A (2014) Biodiesel production from mahua oil by using two-step trans-esterification process. Chem Sci Rev Lett 3(9):52–57
Zaher FA, Soliman HM (2015) Biodiesel production by direct esterification of fatty acids with propyl and butyl alcohols. Egypt J Pet 24(4):439–443
Sathish Kumar R, Suresh Kumar K, Velraj R (2015) Optimization of biodiesel production from Manilkara Zapota (L.) seed oil using Taguchi method. Fuel 140:90–96
Karmakar B, Dhawane SH, Halder G (2018) Optimization of biodiesel production from castor oil by Taguchi design. J Environ Chem Eng 6(2):2684–2695
Dhawane SH, Karmakar B, Ghosh S, Halder G (2018) Parametric optimisation of biodiesel synthesis from waste cooking oil via Taguchi approach. J Environ Chem Eng 6(4):3971–3980
Sudsakorn K, Saiwuttikul S, Palitsakun S, Seubsai A, Limtrakul J (2017) Biodiesel production from Jatropha Curcas oil using strontium-doped CaO/MgO catalyst. J Environ Chem Eng 5(3):2845–2852
Habaki H, Hayashi T, Egashira R (2018) Deacidification process of crude inedible plant oil by esterification for biodiesel production. J Environ Chem Eng 6(12):3054–3060
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sathish Kumar, R., Krupa Vara Prasad, A. Environment friendly butyl ester biodiesel production from mahua oil: optimization and characterization. SN Appl. Sci. 1, 872 (2019). https://doi.org/10.1007/s42452-019-0913-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0913-6