Abstract
The plasticity characteristics of lateritic soil with varying Sporosarcina pasteurii (S. pasteurii) suspension density and compositional variables were evaluated in microbial-induced calcite precipitation (MICP) application. The liquid limit value of the natural lateritic soil was used to prepare samples with three mix proportions of the bacteria and cementation reagent (i.e., 25% bacteria–75% cementation reagent, 50% bacteria–50% cementation reagent and 75% bacteria–25% cementation reagent). The S. pasteurii suspension densities used to trigger the MICP process are 0, 0.5, 2.0, 4.0, 6.0 and 8.0 McFarland standards (i.e., 0, 1.50 × 108, 6.0 × 108, 1.20 × 109, 1.80 × 109 and 2.40 × 109 cells/ml, respectively). Tests carried out on the treated specimens include Atterberg limits and linear shrinkage as well as calcite content using the acid wash method. Results obtained showed a general decrease in the Atterberg limit values with higher S. pasteurii suspension density. The best improvement of plasticity index was achieved for lateritic soil prepared with 75% S. pasteurii and 25% cementation reagent at S. pasteurii suspension density of 2.40 × 109 cells/ml with a corresponding peak 6.0% calcite content. Also, a maximum 4% contaminant concentration of a synthetic leachate produced the best S. pasteurii growth pattern.
Similar content being viewed by others
1 Introduction
The use of deficient or problematic soils in engineering is unavoidable due to increasing urbanization [29, 36]. Deficient soils are generally characterized by their high plasticity characteristics, low strength and high compressibility [67]; such soils are generally not good for engineering use without modification or stabilization. Chemical additives have been conventionally used as soil modification/stabilization agents for over a century. However, in the last decade, there has been an important shift toward “green” and sustainable technologies due to the environmental disquiets linked to the use of chemical additives. A novel and innovative method known as microbial-induced calcite precipitation (MICP) is evolving and is being evaluated by researchers [18, 23, 33, 38, 48, 50, 52, 53, 55,56,57,58,59,60, 66, 73].
The MICP research area is multi-disciplinary in nature and involves microbiologists and civil engineers who aim to provide a soil improvement method that is environmentally friendly and sustainable. MICP is a gentle alternative to the conventional soil improvement methods currently in use which have been adjudged to be environmentally unfriendly [22, 23, 30, 43]. Although recognition has been given to laboratory studies on MICP, the lack of understanding the long-term mechanical properties of bio-cemented soil and its field upscaling has delayed its extensive acceptance for practical engineering applications [28, 42]. The environment-friendly features of the urease producing microorganisms also cause very little or no damage to the soil, human health, as well as the environment since most of them are natives of the soil [55]. Because of their varied ecology, microorganisms exhibit various functional characters that are beneficial in nature and it is fair to say that bacteria can be found almost everywhere, from the friendliest environments to the most hostile setting [32]. One of the processes that MICP relies upon which has also attracted the interest of researchers is bio-cementation which occurs naturally through biochemical processes to produce calcite which is a key parameter on which MICP revolves [43].
Another advantage of MICP is that the reactions between microorganisms and the cementation reagent occur at ambient temperature and therefore require minimum extra energy for the release of carbon underground to mitigate greenhouse gases [23, 33, 40, 67]. Bacteria can be nearly round, rod-like or spiral in shape with cell diameter from 0.5 to 3 μm [37]. Their size could decrease under stress conditions to about 0.2 μm. They are self-replicating in nature and can survive under harsh condition at low to high acidity or salinity [41]. Microorganisms relate with the surrounding environment through enzymatic action, and nearly two-thirds of the resulting products of such interaction are calcium-bearing minerals or calcite minerals required for cementation [74].
An important requirement for effective MICP process is the presence of microorganism which serves as nucleation site. The microorganisms interact with each other and solid surface to form what is known as biomass or biofilm, which is a combination of buildup of single or complex organism cells and extracellular polymeric substances (EPS) [8, 39]. Proto et al. [65] reported that the high specific surface area of soils facilitates the formation of biofilms that significantly have the potential to influence the engineering behavior of soil. Or et al. [54] reported that a gram of EPS matrix can attract up to 15–20 g of water through osmotic and capillary forces which results in the swelling of the matrix. This characteristic of EPS enables it to act as a sponge during MICP processes.
Researchers [5, 21, 27] have used either the microtiter (MtP), Congo red agar (CRA) and/or polymeric chain reaction (PCR) to investigate the formation of biofilms formation. However, it is pertinent to state that not all microorganisms are able to form biofilm. The modification of soils into a more workable construction material using MICP process through bio-cementation is therefore desirable.
According to Neupane [50] and Cardoso et al. [11], coarse-grained soils are good candidates for an effective MICP technique; however, this study was conducted with the aim of understanding the efficacy of MICP technique in low plasticity soils in order to establish the relationship between plasticity characteristics and MICP with different compositional variables (i.e., S. pasteurii suspension density and S. pasteurii suspension–cementation reagent mix ratio). The optimal requirements for different organisms are different in terms of bacterial densities, water requirements and other compositional variables for effective MICP output [48]. Therefore in this study, laboratory experiments were performed using an indigenous microorganism (S. pasteurii) cultured from the lateritic soil to trigger the MICP process by hydrolyzing the urea in the presence of calcium ions and thereby initiating the precipitation of calcite within the soil voids.
The practical application of the study, if the findings are favorable, is to use the technique as a viable alternative for the improvement of low plasticity lateritic soils that do not meet specifications for engineering purposes. The novelty and originality of this study is that most studies conducted relating to MICP focused on strength and hydraulic conductivities of sand (bio-cementation and bio-clogging), while information on the plasticity characteristics of MICP treated residual lateritic soils is very limited. The findings of this research will be practically relevant to engineers involved in geotechnical engineering practice.
2 Materials and methods
2.1 Materials
2.1.1 Soil
The soil sample used in this study is a lateritic soil obtained at depths between 0.5 m and 3.0 m by disturbed sampling method from Abagana (Latitude 6°10′15″N and Longitude 6°58′10″E), Njikoka Local Government Area, Anambra State, Nigeria.
2.1.2 Bacteria
The microorganism used in the study is S. pasteurii isolated and cultured from the lateritic soil collected and grown in the laboratory using the biochemical test kit for Bacillus species.
2.1.3 Cementation reagent
The cementation reagent is composed of 20 g urea, 10 g NH4Cl, 3 g nutrient broth, 2.8 g CaCl2 and 2.12 g NaHCO3 per liter of de-ionized water reported in several studies (e.g., [4, 12, 23, 38, 63, 69, 71, 72]. In all the studies cited, 3 g/l of nutrient broth was added to the cementation reagent because it was the most viable amount for survival of bacteria [66]. Urea was added aseptically after autoclaving the other solutions in the cementation reagent to avoid it decomposition by heat [62].
2.2 Methods
2.2.1 Bacteria isolation, culture/growth medium
The bacteria used in this study are Sporosarcina pasteurii, which was isolated and cultured from the lateritic soil collected from an erosion prone site in Abagana community in Anambra state, Nigeria. The conventional method was used to identify the bacteria. Sporosarcina pasteurii was isolated from the soil inoculated on Ammonium-Yeast Extract media (i.e., 20 g yeast extract, 10 g ammonium chloride, 2 g urea and 0.1 g nickel per liter of distilled water under aerobic condition), and NaOH was used to adjust the pH of the media to 9.0 [26, 46]. The media was transferred in an aliquot amount of 10 ml per culture bottle corked with aluminum foil paper and autoclaved at 121 °C per 1.1 kg pressure for 15 min. With the aid of a sterilized scoop, 1 g each of the soil collected from the soil sampling points was inoculated into each of the culture bottle and incubated at 37 °C for 24 48 h to facilitate proper isolation, identification and characterization of S. pasteurii. Biochemical confirmatory tests were carried out on the test organisms using procedures described by Cheesbrough [14]. Pure culture of the isolates was stored in a freezer at a temperature below 4 °C and retrieved for use when needed. The isolates were sub-cultured every two months.
2.3 Characterization of S. pasteurii
2.3.1 Phenotypic tests
The following are some of the morphological tests conducted to identify and characterize microorganisms: colony, gram staining reaction, urease production, spore staining test, motility test, indole test, Voges Proskauer test, methyl red test, citrate utilization, starch hydrolysis, casein production, catalase production, hydrogen sulfide production, ammonium sulfate precipitation and effect of nickel [61] and biofilm formation using Congo red agar [27]. The tests were conducted using a biochemical test kit for Bacillus species to which S. pasteurii belongs.
2.3.2 Preparation of synthetic leachate
The synthetic leachate used in this study was prepared by mixing 250 ml of distilled water with CuSO4, PbNO3 and Cd in five concentrations in the range 1–5% by weight per volume. The organism was inoculated into each concentration, and the growth pattern of S. pasteurii was monitored for 30 days using spectrophotometer.
2.3.3 Viscosity and torque
The viscosity and torque for the various S. pasteurii suspension densities and the cementation reagent were measured using RV DV-1 Brookfield digital viscometer using the procedure described by Hassan et al. [31].
2.3.4 McFarland standards
McFarland standards are turbidity standards that are used to gauge approximately how many bacteria are present in a liquid suspension. The standards are used to visually compare the turbidity of a suspension of bacteria with the turbidity of the appropriate standard. The reagents that make up each of the Standards are shown in Table 1 [13]. The volumes of the two reagents are adjusted to prepare standards of different turbidity that represent different suspension densities of the bacteria. For an organism that has a faster growth rate, 0.1 ml is inoculated into prepared growth media of desired quantity (i.e., either nutrient broth or peptone broth) before incubation. If the organism has a slow growth rate, 0.5 ml is inoculated into the same growth media of desired quantity and then incubated for between 24 and 48 h. The highest growth for the bacterial suspension is equivalent to the McFarland standard which is equivalent to the bacterial suspension density.
2.3.5 MICP treatment
Two hundred and fifty grams each of the air-dried soil sample passing through British Standard (BS) No. 40 sieve (425 µm aperture) was treated with the 44.0% liquid limit value of the natural soil in three mix proportions, namely: 11%:33% representing 25% bacteria–75% cementation reagent, 22%:22% representing 50% bacteria–50% cementation reagent and 33%:11% representing 75% bacteria–25% cementation reagent. The mix proportions were used to determine the bacteria–cementation reagent combination that produced the best improvement in the plasticity characteristics of the microbial-induced calcite precipitate treated soil. Different S. pasteurii suspension densities of 0, 0.5, 2.0, 4.0, 6.0 and 8.0 McFarland standards (i.e., equivalent to 0, 1.50 × 108, 6.0 × 108, 1.20 × 109, 1.80 × 109 and 2.40 × 109 cells/ml, respectively) were used to trigger the MICP process, respectively. The treated specimens were dried under laboratory condition as described by Osinubi et al. [56,57,58,59,60] to allow the MICP process alter the soil properties before Atterberg limits, and linear shrinkage tests were carried out in accordance with the procedures outlined in BS 1377 [10]. The results reported in this study are average of three tests.
2.3.6 Calcite content
The acid washing method was used to determine the calcite content. Five grams of the treated soil was mixed with 20 ml of 2 M hydrochloric acid (HCl) to dissolve calcium carbonate on the specimen that recorded the highest reduction in plasticity index treated with the bacteria–cementation reagent mix proportions considered. Thereafter, the solution and insoluble soil solid were washed with distilled water on a filter paper with a coarse pore size in a No. 200 sieve for 10 min during which soluble calcium was removed from the soil particles. Subsequently, the solid particles retained on the sieve were oven-dried at a temperature of 105 °C and weighed. The difference between the weight of the originally treated soil sample (A) and the washed soil sample (B) was taken as the mass of calcium carbonate (calcite). The calcite content (CC) in percentage was calculated using Eq. (1) [19, 68]:
2.3.7 Microanalysis
Microanalysis using scanning electron microscope (SEM) was carried out on the natural and the treated lateritic soil to investigate the changes in morphological features due to the formation and distribution of calcite bonds on the inter particle surface in the microstructure of the soil. The test was carried out with a Phenom World Pro desktop SEM equipped with a software application that could automate data collection and image interpretation. The specimen was placed in a motorized tilt and rotation sample holder which was controlled by a dedicated motion control pro suite application which initiates an endless 360° rotation with a pseudo-eucentric tilting adapted focus ranging from 10° to 45°, which allows the production of accurate size information from micro- and nano-fiber samples [64].
3 Results and discussion
3.1 Index properties
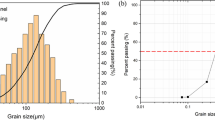
The natural lateritic soil is classified as an A-4 (3) soil in the AASHTO classification system [1] and SC in the Unified Soil Classification System (USCS) [7]. A summary of the physical properties of the natural soil is given in Table 2. X-Ray diffraction results showed that kaolinite is the dominant clay mineral in the natural lateritic soil. Ombaka [51] reported that the arrangement in kaolinite has strong binding forces between the layers that resist expansion when they become wet, which in addition to the measured cation exchange capacity (CEC) value of 5.50 meq/100 g may have been responsible for the low plasticity, shrinkage and swelling behavior exhibited. Similar findings were reported by Murray [49], Aroke et al. [6] and Kamtchueng et al. [35]. The particle size distribution curve of the natural soil is shown in Fig. 1.
The mineralogical composition of the soil summarized in Table 3 indicates that the silica–sesquioxide ratio value {i.e., SiO2/(Al2O3 + Fe2O3)} of 1.64 is between 1.33 and 2.00 recommended for lateritic soil [9, 34]. The three oxides (i.e., SiO2, Al2O3 and Fe2O3) recommended by Bell [9] for the classification of residual soils into laterite, lateritic soil and non-lateritic soil constitute about 90.91% (see Table 3) of the soil’s mineralogical composition. The enhancement of these oxides in the soil could be linked to chemical weathering of the parent rock. Although Al2O3 and Fe2O3 constituted about 34.41% of the mineralogical composition of the soil, their effect on the test organism was not investigated in this study. However, Meyer et al. [44] reported that the interaction of Fe2O3 with S. pasteurii in the presence of water produced more hardened layer of the soil due to the precipitation of calcite, thus making its presence in the soil to be a viable component in MICP process. Also, Sun et al. [70] reported that Al2O3 is an excellent S. pasteurii carrier that enhances the precipitation of calcite during MICP process. The low loss on ignition (LOI) value (see Table 3) is indicative of low-level organic matter, and water content as well as impurities in the soil sample.
A summary of the elemental composition of the natural lateritic soil is given in Table 4. The highest elemental concentration was recorded for iron followed by aluminum and silicon. The mineralogical concentrations of the three elements presented in Table 3 were used to classify the soil as described above.
3.2 Characterization of S. pasteurii and viscosity of additives used
A summary of the confirmatory tests results used to characterize the organism using biochemical test kit for Bacillus species as well as the viscosity of the additives used to treat the lateritic soil is given in Tables 5 and 6, respectively. The results are consistent with the requirement for MICP processes reported by Achal and Pan [2].
3.3 Atterberg limits
Atterberg limits (i.e., liquid limit (LL), plastic limit (PL) and plasticity index (PI)) were used to evaluate the plastic behavior of soils in connection to the amount of water content in the soil as regards its transition from solid to liquid phase. The effect of different mix proportions of S. pasteurii and cementation reagent for different S. pasteurii suspension densities on Atterberg limits of the lateritic soil is shown in Figs. 2, 3 and 4.
3.3.1 Liquid limit
Generally, liquid limit (LL) values initially decreased to minimum values before increasing with higher S. pasteurii suspension density regardless of the bacteria–cementation reagent mix proportion considered. The LL values initially decreased from 44% for the natural soil to minimum values of 37.6%, 39.4% and 36.5% at S. pasteurii suspension densities of 1.50 × 108/ml, 6.0 × 108/ml and 1.80 × 109/ml for 25% bacteria–75% cementation reagent, 50% bacteria–50% cementation reagent and 75% bacteria–25% cementation reagent, respectively (see Fig. 2). Thereafter, the LL values for the three mix proportions increased to 39.2%, 39.6% and 38%, respectively, at S. pasteurii suspension density of 2.40 × 109/ml. The specimen treated with 75% bacteria–25% cementation reagent mix recorded the best LL value of 36.5% at S. pasteurii suspension density of 1.80 × 109/ml, which can be attributed to the high bacteria content of that enhanced the precipitation of more calcite compared to the other two mix proportions with lower bacteria contents.
3.3.2 Plastic limit
Plastic limit (LL) values generally decreased with increased S. pasteurii suspension density regardless of the bacteria–cementation reagent considered. The PL values decreased from 21.55% for the natural soil to minimum values of 15.2%, 18.3% and 17.6% at 2.40 × 109, 6.0 × 108 and 1.80 × 109 cells/ml for 25% bacteria–75% cementation reagent, 50% bacteria–50% cementation reagent and 75% bacteria–25% cementation reagent, respectively. The results show that the PL of lateritic soil can be greatly reduced with 25% bacteria–75% cementation reagent mix proportion with a minimum S. pasteurii suspension density of 1.50 × 108 cells/ml.
3.3.3 Plasticity index
The effect of different proportions of S. pasteurii and cementation reagent at different S. pasteurii suspension densities on the plasticity index (PI) of lateritic soil is shown in Fig. 4. The PI values generally decreased from 22.5% for the natural soil to 20.6%, and 19.5%, at 1.50 × 108 cells/ml and 17.8% at 2.40 × 109 cells/ml suspension density for 25% bacteria–75% cementation reagent–50% bacteria–50% cementation reagent and 75% bacteria–25% cementation reagent mix proportions, respectively. It is pertinent to state that decrease in PI value is desirable for any method adopted for the improvement of soil for engineering use. Therefore, results obtained suggest that the soil improvement technique used in this study is appropriate. The improvement of PI of the lateritic soil can be ranked by performance of the mix proportions in the order 75% bacteria–25% cementation reagent > 50% bacteria–50% cementation reagent > 25% bacteria–75% cementation reagent.
The decrease in PI values could be due to the precipitation of calcite in the voids of the soil matrix. Furthermore, cation exchange reactions could have occurred between clay minerals within the soil and calcite formed, since the combined composition of the soil–calcite mixture contained multivalent cations Ca2+, Al3+, Fe3+, etc., that promote flocculation of clay particles as reported by Coka [20], Eberemu [24] and Fehervari et al. [25]. The results recorded in this study are consistent with the findings reported by Osinubi et al. [55] and Moravej et al. [45], but contradict the findings reported by Neupane [50].
3.4 Linear shrinkage
The effect of different mix proportions of S. pasteurii–cementation reagent mix proportions for different S. pasteurii suspension densities on linear shrinkage (LS) of lateritic soil is shown in Fig. 5. Generally, LS values initially decreased to minimum values with increased S. pasteurii suspension density regardless of the bacteria–cementation reagent mix proportion considered. Thereafter, LS values marginally increased before finally decreasing to least values recorded at S. pasteurii suspension density of 2.40 × 109/ml. The LS values decreased from 9.0% for the natural soil to 6.9%, 6.3% and 6.4% for 25% bacteria–75% cementation reagent, 50% bacteria–50% cementation reagent and 75% bacteria–25% cementation reagent mix proportions, respectively, at S. pasteurii suspension density of 2.40 × 109/ml.
3.5 Calcite content
The variation of calcite content of lateritic soil with S. pasteurii suspension density for the different bacteria–cementation mix proportions considered is shown in Fig. 6. Calcite content (CC) generally increased with higher S. pasteurii suspension density for the mix proportions considered. However, the CC values increased from 1.6% for the natural soil to peak value of 5.0% for specimen treated with 50% bacteria–50% cementation reagent mix proportion at S. pasteurii suspension density of 1.20 × 109 cells/ml as well as 4.0% and 6.0% for specimens treated with 25% bacteria–75% cementation reagent and 75% bacteria–25% cementation reagent mix proportions, respectively, at S. pasteurii suspension density of 1.80 × 109 cells/ml. The specimen treated with 75% bacteria–25% cementation reagent mix proportion recorded the highest calcite content of 6.0% at minimum S. pasteurii suspension density of 1.80 × 109/ml because more organisms were available for the hydrolysis of the urea present in the cementation reagent to precipitate higher calcite content compared to other mix proportions.
3.6 Toxicity effect of metals on S. pasteurii
The variation of colorimetric growth rate of S. pasteurii with time when inoculated in the synthesized leachate is shown in Fig. 7. It was observed that for contaminant concentrations in the 1–3% range, the growth pattern of the organisms was log-normal, exponential and constant up to the 18th day, but reduced from the 21st day for 1% and 2% contaminant concentration, while 3% contaminant concentration recorded the second stage of exponential growth from the 24th day and remained constant up to the 30th day. The 4% and 5% contaminant concentrations initially recorded exponential growth patterns that later became constant, but 5% concentration recorded a decrease from the 21st day to the end of the test period. The results obtained indicate that 4% contaminant concentration gave the best growth for the organism in agreement with results presented in Table 6. Similar results were reported by Stock-Fischer et al. [69], Mugwar and Harbottle [47] as well as Adharsh et al. [3].
3.7 Microanalysis
Scanning electron microscope (SEM) was used to examine the soil texture, particle shapes and to confirm calcite precipitation at microscale on the lateritic soil. Figure 8a, b shows the micrographs of specimens of the natural soil and the treated lateritic soil used for the Atterberg limits tests. It can be observed that the microcracks/pores in the natural soil are sealed in the treated soil because of the reaction between the microorganisms and the cementation reagent in the soil. Qualitatively, the sealing of the cracks/pores underscores bio-cementation/bio-clogging process during the treatment of the natural soil in agreement with the findings reported by Cheng and Cord-Ruwisch [15] and Cheng et al. [16, 17].
4 Conclusion
From the results of the laboratory tests results conducted on lateritic soil classified as A-4(3) or SC in the AASHTO and USCS classification system, respectively, treated with 25% bacteria–75% cementation reagent, 50% bacteria–50% cementation reagent and 75% bacteria–25% cementation reagent mix proportions, the following conclusions can be made:
-
1.
MICP treatment of the natural lateritic soil generally improved its Atterberg limits (LL, PL and PI) with 75% bacteria–25% cementation reagent mix proportion producing the best result at S. pasteurii suspension density of 2.40 × 109 cells/ml.
-
2.
Maximum calcite content of 6.0% was precipitated at minimum S. pasteurii suspension density of 1.80 × 109 cells/ml.
-
3.
Based on the results of the study carried out, S. pasteurii is effective in improving the plasticity characteristics of the lateritic soil used in the MICP application considered.
-
4.
A maximum 4% contaminant concentration of a synthetic leachate produced the best S. pasteurii growth pattern.
5 Recommendation
Based on the results obtained in the study, 75% bacteria–25% cementation reagent mix proportion at S. pasteurii suspension density of 2.40 × 109 cells/ml can be used to improve the plasticity characteristics of A-4(3) or SC soil for engineering purposes.
References
AASHTO (1986) Standard specifications for transport materials and methods of sampling and testing, 14th edn. American Association of State Highway and Transport Officials (AASHTO), Washington, DC
Achal V, Pan X (2011) Characterization of urease and carbonic anhydrase producing bacteria and their role in calcite precipitation. Curr Microbiol 62:894–902. https://doi.org/10.1007/s00284-010-9801-4
Adharsh R, Stephen W, Raju S, Jonathan B, Eduardo M-R, Charles KSM (2018) Biomineralisation performance of bacteria isolated from a landfill in China. Can J Microbiol (CJM) 64(12):945–953
Al Qabany A, Mortensen B, Martinez B, Soga K, Dejong J (2011) Microbial carbonate precipitation: correlation of S-wave velocity with calcite precipitation. In: Proceedings geo frontiers in geotechnical engineering 2011: technical papers, ASCE, 3993-4001
Arciola CR, Campoccia D, Baldassarri L, Donati ME, Pirini V, Gamberini S, Montanaro L (2006) Detection of biofilm formation in Staphylococcus epidermidis from implant infections. Comparison of a PCR-method that recognize the presence of ica genes with two classic phenotypic methods. J Biomed Mater Res A 76:425–430
Aroke UO, El-Nafaty UA, Osha OA (2013) Properties and characterization of Kaolin Clay from Alkaleri, North-Eastern Nigeria. Int J Emerg Technol Adv Eng 3(11):387–392
ASTM (1992) Annual book of standards, vol 04.08. American Society for Testing and Materials, Philadelphia
Bai Y, Guo X, Li Y, Huang T (2017) Experimental and visual research on the microbial induced carbonate precipitation by Pseudomonas aeruginosa. AMB Expr 7(1–9):57. https://doi.org/10.1186/s13568-017-0358-5
Bell FG (1993) Engineering geology. Blackwell Scientific Publications Oxford, London, p 104
BS 1377 (1990) Method of testing soils for civil engineering purpose. British Standard Institute, BSI, London
Cardoso R, Pires IS, Duarte OD, Monteiro GA (2018) Effects of clay’s chemical interactions on biocementation. Appl Clay Sci 156:96–103. https://doi.org/10.1016/j.clay.2018.01.035
Chahal N, Rajor A, Siddique R (2011) Calcium carbonate precipitation by different bacterial strains. Afr J Biotechnol 10(42):8359–8372. https://doi.org/10.5897/AJB11.345
Chapin KC, Lauderdale T (2003) Reagents, stains, and media: bacteriology. In: Murray PR, Baron EJ, Jorgensen JH, Faller MAP, Yolken RH (eds) Manual of clinical microbiology, 8th edn. ASM Press, Washington, DC, p 358
Cheesbrough M (2006) District laboratory practice in tropical countries part 2, 2nd edn. Cambridge University Press, New York
Cheng L, Cord-Ruwisch R (2012) In situ soil cementation with ureolytic bacteria by surface percolation. Ecol Eng 42:54–72. https://doi.org/10.1016/j.ecoleng.2012.01.013
Cheng L, Cord-Ruwisch R, Shahin MA (2013) Cementation of sand soil by microbially induced calcite precipitation at various saturation degrees. Can Geotech J 50:81–90
Cheng L, Shahin MA, Cord-Ruwisch R (2017) Surface percolation for soil improvement by bio-cementation utilizing in situ enriched indigenous aerobic and anaerobic ureolytic soil microorganisms. Geomicrobiol J 34(6):546–556. https://doi.org/10.1080/01490451.2016.1232766
Chittoori BCS, Burbank M, Islam MT (2018) Evaluating the effectiveness of soil-native bacteria in precipitating calcite to stabilize expansive soils. ASCE IFCEE 2018 Geotech Special Publ 296:59–68. https://doi.org/10.1061/9780784481592.007
Choi S, Park S, Wu S, Chu J (2017) Methods for calcium carbonate content measurement of biocemented soils. ASCE J Mater Civ Eng 29(11):06017015. https://doi.org/10.1061/(asce)MT.1943-5533.0002064
Coka E (2001) Use of class C fly ashes for the stabilization of an expansive soil. ASCE J Geotech Geoenvironmental Eng 127(7):568–573
Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, Beachey EH (1985) Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol 22:996–1006
Dawoud O, Chen CY, Soga K (2014) Microbial induced calcite precipitation for geotechnical and environmental applications. In: Proceedings new frontiers in geotechnical engineering 2014: technical papers, vol 234. ASCE, Geotechnical Special Publication, pp 11–18
Dejong JT, Fritzges MB, Nusslein K (2006) Microbially induced cementation to control sand response to undrain shear. ASCE J Geotech Geoenvironmental Eng 132(11):1381–1392. https://doi.org/10.1061/(ASCE)1090-0241(2006)132:11(1381)
Eberemu AO (2013) Evaluation of bagasse ash treated lateritic soil as a potential barrier material in waste containment application. Acta Geotech. https://doi.org/10.1007/s11440-012-0204-5
Fehervari A, Gates WP, Patti AF, Turney TW, Bouazza A, Rowe RK (2016) Potential hydraulic barrier performance of cyclic organic carbonate modified bentonite complexes against hyper-salinity. Geotext Geomembr 44:748–760. https://doi.org/10.1016/j.geotexmem.2016.06.002
Feng K, Montoya BM, Evans TM (2014) Numerical investigation of microbial induced cemented sand mechanical behaviour. In: Proceedings geo-congress 2014: technical papers, vol 234. ASCE, Geotechnical Special Publication, pp 1644–1653
Freeman DJ, Falkiner FR, Keane CT (1989) New method for detecting slime production by coagulase-negative staphylococci. J Clin Pathol 42:872–874
Gao Y, Hang L, He J, Chu J (2018) Mechanical behaviour of biocemented sands at various treatment levels and relative densities. Acta Geotech. https://doi.org/10.1007/s11440-018-0729-3
Golakiya HD, Savani CD (2015) Studies on geotechnical properties of black cotton soil stabilized with furnace dust and dolomite lime. Int Res J Eng Technol (IRJET) 2(8):810–823
Hamdan N, Kavazanjian E Jr, Rittmann BE, Karatas I (2016) Carbonate mineral precipitation for soil improvement through microbial denitrification. Geomicrobiol J. https://doi.org/10.1080/01490451.2016.1154117
Hassan AB, Abolarin MS, Nasir A, Mshelia SG (2006) Fabrication and testing of viscosity measuring instrument (viscometer). Leonardo Electron J Pract Technol 5(8):49–57
Helden JV, Toussaint A, Thieffry D (eds) (2012) Bacterial molecular networks: methods and protocols. Springer, New York. ISBN 978-1-61779-360-8
Ivanov V, Chu J (2008) Application of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev Environ Sci Biotechnol 7(2):139–153. https://doi.org/10.1007/s11157-007-9126-3
Joachim AWR, Kandiah S (1941) The compositions of some local concretions and clays. Trop Agric 96:67–75
Kamtchueng BT, Onana VL, Fantong WY, Ueda A, Ntouala RFD, Wongolo MHD, Ndongo GB, Ze ANO, Kamgang VK, Ondoa JM (2015) Geotechnical, chemical and mineralogical evaluation of lateritic soils in humid tropical area (Mfou, Central-Cameroon): implications for road construction. Int J Geo-Eng 6(1):1–21. https://doi.org/10.1186/s40703-014-0001-0
Kang C, Kwon Y, So J (2016) Soil bioconsolidation through microbially induced calcite precipitation by Lysinibacillus sphaericus. WJ-8. Geomicrobiol J 33(6):473–478. https://doi.org/10.1080/01490451.2015.1053581
Kayser FH, Bienz KA, Eckert J, Zinkernagel RM (2005) Medical microbiology, 10th edn. Thieme, New York
Kim G, Youn H (2016) Microbially induced calcite precipitation employing environmental isolates. Materials 9(468):1–10. https://doi.org/10.3390/ma9060468
Lin H (2016) Microbial modification of soil for ground improvement. An unpublished PhD Theses and Dissertations. 2687. http://preserve.lehigh.edu/etd/2687. Accessed 11 Aug 2018
Lin H, Suleiman MT, Helm J, Brown DG (2014) Measurement of bonding strength between glass beads treated by microbial-induced calcite precipitation (MICP). In: Proceedings geo-congress 2014: technical papers, vol 234. ASCE, Geotechnical Special Publication, pp 1625–1634
Madigan MT, Martinko JM, Dunlap PV, Clark DP (2008) Brock biology of microorganisms, 12th edn. Benjamin Cummings, San Francisco
Mahawish A, Bouazza A, Gates WP (2019) Factors affecting the bio-cementing process of coarse sand. Ground Improv 172(1):25–36. https://doi.org/10.1680/jgrim.17.00039
Mahawish A, Bouazza A, Gates WP (2019) Unconfined compressive strength and visualization of the microstructure of coarse sand subjected to different bio-cementation levels. J Geotech Geoenvironmental Eng 145(8):04019033. https://doi.org/10.1061/(asce)gt.1943-5606.0002066
Meyer FD, Bang S, Min S, Stetler LD, Bang SS (2011) Microbiologically-induced soil stabilization: application of Sporosarcina pasteurii for fugitive dust control. In: ASCE geo-frontiers, pp 4002–4011
Moravej S, Habibagahi G, Nikooee E, Niazi A (2018) Stabilization of dispersive soils by means of biological calcite precipitation. Geoderma 315(2018):130–137. https://doi.org/10.1016/j.geoderma.2017.11.037
Mortenson BM, Haber MJ, Dejong JT, Caslake LF, Nelson DC (2011) Effects of environmental factors on microbial-induced calcite precipitation. Appl Microbiol 111(2):338–349. https://doi.org/10.1111/j1365-2672-2011.05065x
Mugwar AJ, Harbottle MJ (2016) Toxicity effects on metal sequestration by microbially-induced carbonate precipitation. J Hazard Mater 314(2016):237–248. https://doi.org/10.1016/j.jhazmat.2016.04.039
Mujah D, Cheng L, Shahin MA (2019) Microstructural and geo-mechanical study on bio-cemented sand for optimization of MICP process. J Mater Civ Eng 31(4):04019025. https://doi.org/10.1061/(ASCE)MT.1943-5533.0002660
Murray HH (1999) Applied clay mineralogy today and tomorrow. Clay Miner 34(1):39–49. https://doi.org/10.1180/000985599546055
Neupane S (2016) Evaluating the suitability of microbial induced calcite precipitation technique for stabilizing expansive soils. An unpublished thesis submitted to Boise State University
Ombaka O (2016) Characterization and classification of clay minerals for potential applications in Rugi Ward, Kenya. Afr J Environ Sci Technol 10(11):415–431. https://doi.org/10.5897/AJEST2016.2184
Omoregie AI, Khoshdelnezamiha G, Senian N, Ong DE, Nissom PM (2017) Experimental optimisation of various cultural conditions on urease activity for isolated Sporosarcina pasteurii strains and evaluation of their biocement potentials. Ecol Eng 109:65–75. https://doi.org/10.1016/j.ecoleng.2017.09.012
Omoregie AI, Siah J, Pei BCS, Yie SPJ, Weissmann LS, Enn TG, Rafi R, Zoe THY, Mkwata HM, Sio CA, Nissom PM (2018) Integrating biotechnology into geotechnical engineering: a laboratory exercise. Trans Sci Technol 5(2):76–87
Or D, Phutane S, Dechesne A (2007) Extracellular polymeric substances affecting pore-scale hydrologic conditions for bacterial activity in unsaturated soils. Vadose Zone J 6(2):298–305
Osinubi KJ, Eberemu AO, Ijimdiya ST, Yakubu SE, Sani JE (2017). Potential use of B. Pumilus in microbial-induced calcite precipitation improvement of lateritic soil. In: Proceedings of the 2nd symposium on coupled phenomena in environmental geotechnics (CPEG2), session: clean-ups, paper, vol 64. Leeds, pp 1–6
Osinubi KJ, Eberemu AO, Ijimdiya TS, Gadzama EW, Yakubu SE (2018a) Improvement of the strength of lateritic soil treated with Sporosarcina pasteurii-induced precipitate. In: 2018 Nigerian building and road research institute international conference. Theme: sustainable development goals (SDGs) and the Nigerian construction industry—challenges and the way forward. Abuja
Osinubi KJ, Gadzama EW, Eberemu AO, Ijimdiya TS (2018b) Volumetric shrinkage of compacted lateritic soil treated with Sporosarcina pasteurii. In: Proceedings of the 1st international civil engineering conference (ICEC 2018) infrastructure development in the context of contemporary economic challenges. Federal University of Technology, Minna, pp 184–193
Osinubi KJ, Yohanna P, Eberemu AO, Ijimdiya TS (2019a) Evaluation of hydraulic conductivity of lateritic soil treated with Bacillus Coagulans for use in waste containment applications. In: Zhan L, Chen Y, Bouazza A (eds) Proceedings of the 8th international congress on environmental geotechnics (ICEG 2018). Towards a sustainable geoenvironment, vol 3. Springer, Hangzhou, pp 401–409. https://doi.org/10.1007/978-981-13-2227-3_50
Osinubi KJ, Sani JE, Eberemu AO, Ijimdiya TS, Yakubu SE (2019b) Unconfined compressive strength of Bacillus Pumilus treated lateritic soil. In: Zhan L, Chen Y, Bouazza A (eds) Proceedings of the 8th international congress on environmental geotechnics (ICEG 2018). Towards a sustainable geoenvironment, vol 3. Springer, Hangzhou, pp 410–418. https://doi.org/10.1007/978-981-13-2227-3_51
Osinubi KJ, Gadzama EW, Eberemu AO, Ijimdiya TS, Yakubu SE (2019c) Evaluation of the strength of compacted lateritic soil treated with Sporosarcina pasteurii. In: Zhan L, Chen Y, Bouazza A (eds) Proceedings of the 8th international congress on environmental geotechnics (ICEG 2018). Towards a sustainable geoenvironment, vol 3. Springer, Hangzhou, pp 419–428. https://doi.org/10.1007/978-981-13-2227-3_52
Owuama CI (2015) Laboratory manual for microbiology. Microtrend Digital Press Nigeria Limited. ISBN 978-978-943-329-2
Pakbaz MS, Behzadipour H, Ghezelbash GR (2018) Evaluation of shear strength parameters of sandy soils upon microbial treatment. Geomicrobiol J. https://doi.org/10.1080/01490451.2018.1455766
Park S, Choi S, Kim W, Lee J (2014a) Effect of microbially induced calcite precipitation on strength of cemented sand. In: Proceedings new frontiers in geotechnical engineering 2014: technical papers, vol 234. ASCE, Geotechnical Special Publication, pp 47–56
Phenom World (2017) Fibermetric. https://www.phenom-world.com/software/fibermetric. Accessed 17 Sept 2018
Proto CJ, DeJong JT, Nelson DC (2016) Biomediated permeability reduction of saturated sands. J Geotech Geoenvironmental Eng 142(12):04016073. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001558
Sharma A, Ramkrishnan R (2016) Study on effect of microbial induced calcite precipitates on strength of fine grained soils. Perspect Sci 8:198–202. https://doi.org/10.1016/j.pisc.2016.03.017
Soon N, Lee L, Khun T, Ling H (2012) An overview of the factors affecting microbial-induced calcite precipitation and its potential application in soil improvement. Int J Civ Environ Struct Constr Archit Eng 6(2):188–194
Soon N, Lee L, Khun T, Ling H (2014) Factors affecting improvement in engineering properties of residual soil through microbial-induced calcite precipitation. ASCE J Geotech Geoenvironmental Eng 140(5):04014006. https://doi.org/10.1061/(asce)gt.1943-5606.0001089
Stocks-Fischer S, Galinat JK, Bang SS (1999) Microbiological precipitation of CaCo3. Soil Biol Biochem 31(11):1563–1571
Sun X, Miao L, Chen R (2019) Adding aluminum oxide to improve the repairing effect of cracks based on bio-remediation. J Adv Concr Technol 17:177–187. https://doi.org/10.3151/Jact.17.4.177
Tirkolaei HK, Bilsel H (2017) Estimation on ureolysis-based microbially induced calcium carbonate precipitation progress for geotechnical applications. Mar Georesour Geotechnol 35(1):34–41. https://doi.org/10.1080/1064119X.2015.1099062
Venkata PN, Velpuri NVP, Yu X, Lee H, Chang W (2016) Influence factors for microbial-induced calcite precipitation in sands. In: Proceedings geo-China 2016: technical papers, vol 263. ASCE Geotechnical Special Publication, pp 44–52
Wang X, Nackenhorst U (2019) A modeling study of the bio-geochemical processes in microbially induced calcite precipitation. In: Zhan L, Chen Y, Bouazza A (eds) Proceedings of the 8th international congress on environmental geotechnics (ICEG 2018). Towards a sustainable geoenvironment, vol 3. Springer, Hangzhou, pp 272–278. https://doi.org/10.1007/978-981-13-2227-3_33
Xu G, Li D, Jiao B, Li D, Yin Y, Lun L, Zhao Z, Li S (2017) Biomineralization of a calcifying ureolytic bacterium microbacterium sp. Electron J Biotechnol 25:21–27. https://doi.org/10.1016/j.ejbt.2016.10.008
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Osinubi, K.J., Eberemu, A.O., Gadzama, E.W. et al. Plasticity characteristics of lateritic soil treated with Sporosarcina pasteurii in microbial-induced calcite precipitation application. SN Appl. Sci. 1, 829 (2019). https://doi.org/10.1007/s42452-019-0868-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0868-7