Abstract
A hydrometallury process was developed to recover the valuable metals from LiNi0.8Co0.15Al0.05O2 cathode materials. In step 1, lithium and aluminum were selectively dissolved by oxalic acid. Utilizing Na2SO3 as the reducing reagent, the reaction was significantly accelerated. The aluminum and lithium in the liquid stream were recovered by chemical precipitation in steps 2 and 3, using KOH and K2CO3 as precipitants, respectively. The mixture of solid Ni and Co oxalates from step 1 was converted to sulfates through acid baking. After dissolution in water, cobalt was recovered as Co2O3 after oxidation and nickel stayed in the solution as NiSO4.
Similar content being viewed by others
1 Introduction
The lithium-ion batteries (LIBs) were widely used in portable electronics, and electric vehicles (EVs) due to their high capacity, excellent cycle life, and high energy density. A significant amount of metal, including lithium, and cobalt, was used for the production of LIBs. In 2018, nearly 80% of the cobalt consumed in China was in rechargeable batteries [1]. With the current growth rate, the LIB global market was estimated to reach $32 billion by 2020 [2]. The supply simply cannot meet the increasing demand. Apart from the supply issues, the spent LIBs cannot be disposed at landfills as they contain heavy metals that pollute the environment [3]. Therefore, the most sensible solution is to recover the valuable metals from spent LIBs. While there are a number of proposed processes for recycling LiCoO2 (LCO) and LiNixMnyCo1 − x − yO2 (LNMC), few are available for LiNi0.8Co0.15Al0.05O2 (LNCA) [4], which is used in Tesla EVs.
Hydrometallurgical processes are commonly used to separate metals from spent LIBs. In most recycling processes, all metals are first dissolved by mineral acids such as hydrochloric acid [5, 6], nitric acid [7, 8], and sulphuric acid [9, 10], or organic acids [11,12,13] such as citric acid [11] and succinic acid [12]. The dissolved metals are then separated by chemical precipitation [14] and/or solvent extraction [15] to recover metal salts in pure form. The organic acids are regarded as the greener alternative than mineral acids; however, it is difficult to recover different metals from the organic acidic medium due to the strong chelation between the acid and metal ions [16]. Hence, only selected metals were dissolved to improve the efficiency of separation [16,17,18]. Specifically, Li et al. [19] used oxalic acid to dissolve lithium from LiNixMnyCo1 − x − yO2 (LNMC) cathode materials. Then, Ni, Co, and Mn were separated by dissolution [20]. Following the same approach, this study develops a novel hydrometallurgy process to separate and recover all the valuable metals from LNCA cathode materials.
2 Experimental and materials
The process flowsheet consists of 6 steps (Fig. 1). Lithium and aluminum are first dissolved by oxalic acid in step 1, producing a liquid stream containing Li+ and Al3+ (stream 3) and a solid stream containing mixed Ni and Co oxalates (stream 4). The aluminum is removed by chemical precipitation using KOH (step 2) while lithium is recovered as lithium carbonate (step 3). The solid mixture of NiC2O4·2H2O and CoC2O4·2H2O in stream 4 is converted to mixed sulfates through an acid baking treatment (step 4). The mixed sulfates (stream 12) are dissolved in water (step 5). The cobalt in the solution is then separated as solid Co2O3 [4] from a solution containing NiSO4 (step 6). These steps are discussed in detail next.
2.1 Selective dissolution by oxalic acid (step 1)
Selective dissolution using oxalic acid was carried out in a 250 mL three-neck round-bottom flask with a magnetic stirrer, a heating mantle, and a reflux condenser at 95 °C for 4 h. The base case dissolution experiment was conducted with 3 g of cathode powder, and 100 mL of 1 M oxalic acid, giving a solid-to-liquid ratio of 35 g/L. 1.58 g of K2SO3 was added as reducing reagent. The effects of reducing reagent concentration, temperature, and solid-to-liquid ratio on dissolution performance were investigated.
2.2 Aluminum removal by chemical precipitation (step 2)
As most of the aluminum was dissolved along with lithium, it had to be removed from the liquid mixture by chemical precipitation. KOH was added in a 20 mL glass vial, and the mixture was agitated with a magnetic stirrer at room temperature. After equilibration for 4 h, the solution and the precipitated solids were separated by vacuum filtration. Collected solids were washed with DDI water and dried in a vacuum oven overnight at 70 °C.
2.3 Lithium recovery by chemical precipitation (step 3)
The chemical precipitation process for lithium recovery was conducted in a 100 mL culture bottle with a magnetic stirrer. 5 M of K2CO3 was added. The mixture was placed in a water bath at 80 °C. After equilibration for 4 h, the liquid solution and the precipitated solids were separated by vacuum filtration. Collected solids were washed with DDI water and dried in a vacuum oven overnight at 70 °C.
2.4 Acid baking of mixed oxalates (step 4) and dissolution (step 5)
The acid baking of mixed oxalates was conducted in a temperature controlled muffle furnace. 3 g of mixed Ni and Co oxalates produced from step 1 was thoroughly mixed with a specific amount of concentrated sulfuric acid (98%) in a porcelain crucible. 1 mL of DDI water was also added to facilitate mixing of the slurry. Then the crucible was transferred to the muffle furnace for a 2-h thermal treatment. The powder from acid baking was dissolved in DDI water in a solid-to-liquid ratio of 50 g/L at a temperature of 70 °C for 3 h. The prepared solution was used as the feed for cobalt precipitation (Stream 14).
2.5 Cobalt precipitation (step 6)
The cobalt precipitation was conducted in a 20 mL glass vial with a magnetic stirrer. A specific volume of NaClO solution was added in 10 mL of feed solution. After equilibration for 4 h, the liquid solution and the precipitated solids were separated by vacuum filtration. Collected solids were washed with DDI water and dried in a vacuum oven overnight at 70 °C.
2.6 Analytical methods
The pH of the solution was measured with a pH/mV meter (Model Mettler- Toledo AG SevenGo2), and the metal concentrations were determined by inductively coupled plasma optical emission spectrometry (ICP-OES) (Model PerkinElmer Optima 7300 DV). The standard solutions were provided by High-Purity Standards, 99.998%, and diluted in double deionized (DDI) water with a resistivity of 18.2 MΩ cm at room temperature. The particle morphology was examined by scanning electron microscope (SEM) (Model JEOL-JSM 7100). The chemical composition of the solids was confirmed with attenuated total reflectance Fourier-transform infrared (ATR-FTIR) spectra (Model Bruker Tensor 27 FTIR with a Bruker Platinum ATR accessory), and thermal gravimetrical analysis (TGA) (Model TA Q5000). The crystal structure was characterized by X-ray diffraction (XRD) system (Model PW1830 Philips, 2KW, Cu anode, graphite monochromator).
3 Results and discussion
3.1 Selective dissolution of lithium and aluminum (step 1)
The effects of various operating parameters including temperature (T), the concentration of reducing reagent (K2SO3), and solid-to-liquid feed ratio (S/L ratio) on this reaction with 3 h of dissolution are discussed next. A summary of the results is presented in Table 1. The second row shows the base case: C = 1 M, T = 95 °C, Creductant = 0.1 M, and S/L = 35 g/L. Considering the solubility of oxalic acid which is 1.1 M at room temperature, the concentration of oxalic acid is selected as 1 M. The third to fifth rows show how the wt% of metal dissolved in stream 3 change in response to the change in a single operating parameter while keeping the remaining two parameters constant at their base case values. Since only trace amounts of Ni and Co were dissolved in stream 3, the following discussion focuses on the concentration change of lithium and aluminum.
3.1.1 Effects of reducing reagent concentration
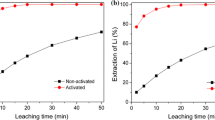
The recovery of Li and Al by selective dissolution under base case conditions with and without K2SO3 as reducing reagent is shown in Fig. 2. Without K2SO3, the dissolution of lithium and aluminum proceeded almost linearly with reaction time. With K2SO3, dissolution was significantly accelerated, reaching the equilibrium values within 1 h. In this study, the reaction time would be fixed at 3 h for further experiments.
When the concentration of K2SO3 was increased to 0.2 M from its base case value of 0.1 M, 3.95% of Ni dissolved in the solution. This was expected because when pH is higher than 1.6, Ni forms a soluble complex with C2O42− ions present in the system.19 When the concentration of K2SO3 was reduced to 0.05 M, the recovery of lithium and aluminum would drop to 87.0% and 73.7% respectively, indicating that there was not enough reducing reagent to react with LNCA cathode materials.
3.1.2 Effect of temperature
The temperature was varied from 65 to 85 °C while keeping other parameters at their base case values (fourth row of Table 1). The experimental results show that when the temperature was decreased from 95 °C (base case value) to 85 °C, the lithium and aluminum recovery decreased from 97.8%, and 91.6% to 95.2%, and 84.2%, respectively. The temperature variation shows a more substantial impact on the dissolution of aluminum than the dissolution of lithium.
3.1.3 Effect of solid-to-liquid feed ratio
The S/L ratio was varied from 30 to 50 g/L (fifth row of Table 1). Experimental results show that 97.8% of lithium and 91.6% of aluminum were dissolved for an S/L ratio of 35 g/L (base case). It dropped to 83.3% and 90.6% for an S/L ratio of 40 g/L and was further reduced to 68.0% and 46.9% at 50 g/L. A better recovery was achieved at the S/L ratio of 30 g/L, giving a recovery of 98.8% for lithium and 96.0% for aluminum. Therefore, an S/L ratio of 30 g/L, instead of the base case value, was further considered below.
3.1.4 Solid characterization
The solid residue needs to be characterized to investigate the chemical reaction happened during dissolution. The solid residue was collected from the selective dissolution using 1 M of oxalic acid, and 0.1 M of K2SO3, with an S/L ratio of 30 g/L, at the temperature of 95 °C. Lithium and aluminum were found in the liquid after selective lithium dissolution as measured by ICP-OES. Nickel and cobalt were present in the solid residue in a ratio of 5.3:1. Moreover, lithium and aluminum account for 0.1% and 0.04% in the solid residue, respectively. The cathode material particles before and after selective dissolution are shown by SEM in Fig. 3. After dissolution, much smaller particles remained. The EDX mapping in Fig. 4 shows that Ni and Co evenly distributed in the particles after dissolution, but no aluminum was detected.
The XRD data of the solid residue is shown in Fig. 5a, which indicates that mixed metals were present [21]. The presence of Ni and Co oxalates was further confirmed by ATR and TGA. Figure 5b shows the ATR spectroscopic results of the solid residue. The bands at 1310 and 1359 cm−1 can be assigned to O–C–O symmetric stretching, and the band at 1610 cm−1 is the result of asymmetric stretching. The broadband at 3370 cm−1 is the fingerprint of hydration in the residue [22,23,24,25]. Figure 5c shows the TGA results obtained for the solid residue in the air at a heating rate of 10 K/min. No weight loss was observed below 150 °C, implying that the powder had no free water. From 150 to 230 °C, the weight loss was about 19.5%, which corresponded to the release of two structural water molecules [26]. The weight loss matched well the theoretical value of 19.5% when H2O was released from a mixture of NiC2O4·2H2O, and CoC2O4·2H2O in the ratio mentioned above. The second stage weight loss resulted from oxalate decomposition [27,28,29].
Taken together, it could be concluded that the liquid phase after selective lithium dissolution was made up of Li+, Al3+, HC2O4−, and H2C2O4. The solid residue was a mixture of CoC2O4·2H2O and NiC2O4·2H2O. The solution (stream 3) after selective dissolution contains 2370 ppm of Li+ and 294 ppm of Al3+.
3.2 Aluminum removal (step 2)
Since K2C2O4 has a higher solubility than Na2C2O4, and (NH4)2C2O4, KOH was selected here as the precipitant. In addition, a concentration of 10 M was used to minimize the dilution of the lithium in the solution, which would affect the lithium recovery afterwards. The results of aluminum removal are listed in Table 2. By adding 0.4 mL of 10 M KOH, 77.8% of Al was precipitated as Al(OH)3. The recovery increased to 99.2% when 0.5 mL of 10 M KOH was used. More was not better. The recovery of Al would drop back to 53.5% by increasing the volume of KOH from 0.5 mL to 0.6 mL. The reason can be understood from Fig. S1, which was simulated using the Medusa software [30] and shows the relation between the solubility of different aluminum species and pH. As the pH of the solution after the selective dissolution is around 1.6, aluminum was in the form of soluble complexes with oxalate ions. When the pH > 12, soluble Al(OH) −4 became the dominant species. At 0.6 mL KOH, the pH was 12.54 (Table 2).
3.3 Lithium recovery (step 3)
After the removal of Al, the solution (stream 7) contained Li + , K + , C2O42−, and OH−. Lithium was recovered by chemical precipitation using K2CO3 as the precipitant at an elevated temperature of 80 °C [31]. By adding 4 mL of 5 M K2CO3 solution to 20 mL of feed solution (stream 7), the concentration of lithium dropped from 2313 to 543 ppm, giving a lithium recovery of 76.5% as lithium carbonate. The XRD and SEM are shown in Fig. 6a and b, respectively, and the purity was checked by ICP, which is > 99.5%.
3.4 Acid baking of the mixed oxalates (step 4) and dissolution (step 5)
The solid stream (stream 4) after selective dissolution contained Co and Ni to be separated. Thermal treatment could be used to process the oxalates. However, the products from the thermal decomposition of NiC2O4·2H2O and CoC2O4·2H2O [21], which are NiO and Co2O3, still need further processing. Instead, an oxidative, thermal treatment using sulfuric acid is used to convert the mixed oxalates to mixed sulfates following the chemical equation below:
Sulfuric acid was selected over other mineral acids because at 337 °C it has the highest boiling point. The amount of sulfuric acid was determined according to the stoichiometry ratio in Eq. 1. The reaction temperature was fixed at 300 °C. The XRD patterns and SEM photo of the solids after thermal treatment is shown in Fig. 7. The XRD pattern of the solids matched well with that of pure NiSO4·2H2O, which is shown in Fig. 7a. The morphology was changed from parallelepipedic to irregular particles, as revealed in Fig. 7b.
3.5 Cobalt precipitation by oxidation (step 6)
The separation of Co and Ni was achieved because of the difference in solubility of Ni(OH)2 (pKs = 14.2) and Co2O3 (pKs = 40.5) [4]. The feed was prepared by dissolving the mixed sulfates in an S/L ratio of 50 g/L, giving an initial pH of 1.83. NaClO was used as an oxidant to oxidize Co2+ to Co3+ [32]. The reaction is shown below:
A series of experiments were conducted to investigate the effects of the molar ratio between NaClO and Co on the metal recovery of Co and Ni, as shown in Fig. 8. As the amount of NaClO increased, the recovery of cobalt was increased from 37.23 to 92.2%. Meanwhile 0.18% and 2.23% of nickel were co-precipitated at the same two ratios. As most of cobalt was precipitated, the remaining nickel in the solution can be recovered as Ni(OH)2 by adding KOH.
4 Conclusion
A process to recover all the valuable metals from LNCA cathode materials was developed. In step 1, selective dissolution using oxalic acid was conducted. The conditions under which the maximum lithium and aluminum dissolved from the cathode materials were identified. Then, the Al3+ and Li+ in the liquid stream were recovered separated by chemical precipitation in step 2 and step 3, using KOH and K2CO3, respectively. The solid mixture from step 1 underwent an acid baking treatment to produce a mixture of nickel and cobalt sulfates. The sulfate mixture was dissolved in water and then separated using NaClO.
Cathode materials with relatively low aluminum concentration were used as the feed in this paper. If cathode on an aluminum foil is considered as the feed, the aluminum in the current collector would give a much higher concentration of aluminum in the solution stream, which might lead to a formation of gel like Al(OH)3. Additional work still needs to be pursued to solve such issue.
References
Jaskula BW (2018) Mineral commodity summaries 2018. U.S. Geological Survey, Reston
Palacin MR, de Guibert A (2016) Why do batteries fail? Science 351(6273):1253292. https://doi.org/10.1126/science.1253292
Chagnes A, Pospiech B (2013) A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries. J Chem Technol Biotechnol 88(7):1191–1199. https://doi.org/10.1002/jctb.4053
Joulié M, Laucournet R, Billy E (2014) Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries. J Power Sources 247:551–555. https://doi.org/10.1016/j.jpowsour.2013.08.128
Zhang P, Yokoyama T, Itabashi O, Suzuki TM, Inoue K (1998) Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries. Hydrometallurgy 47(2):259–271. https://doi.org/10.1016/S0304-386X(97)00050-9
Castillo S, Ansart F, Laberty-Robert C, Portal J (2002) Advances in the recovering of spent lithium battery compounds. J Power Sources 112(1):247–254
Lee CK, Rhee K-I (2003) Reductive leaching of cathodic active materials from lithium ion battery wastes. Hydrometallurgy 68(1):5–10. https://doi.org/10.1016/S0304-386X(02)00167-6
Lee CK, Rhee K-I (2002) Preparation of LiCoO2 from spent lithium-ion batteries. J Power Sources 109(1):17–21
Zou H, Gratz E, Apelian D, Wang Y (2013) A novel method to recycle mixed cathode materials for lithium ion batteries. Green Chem 15(5):1183–1191. https://doi.org/10.1039/c3gc40182k
Ferreira DA, Prados LMZ, Majuste D, Mansur MB (2009) Hydrometallurgical separation of aluminium, cobalt, copper and lithium from spent Li-ion batteries. J Power Sources 187(1):238–246. https://doi.org/10.1016/j.jpowsour.2008.10.077
Li L, Ge J, Wu F, Chen R, Chen S, Wu B (2010) Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant. J Hazard Mater 176(1):288–293
Li L, Qu W, Zhang X, Lu J, Chen R, Wu F, Amine K (2015) Succinic acid-based leaching system: a sustainable process for recovery of valuable metals from spent Li-ion batteries. J Power Sources 282:544–551
Nayaka GP, Pai KV, Santhosh G, Manjanna J (2016) Dissolution of cathode active material of spent Li-ion batteries using tartaric acid and ascorbic acid mixture to recover Co. Hydrometallurgy 161:54–57. https://doi.org/10.1016/j.hydromet.2016.01.026
Cai G, Fung KY, Ng KM, Wibowo C (2014) Process development for the recycle of spent lithium ion batteries by chemical precipitation. Ind Eng Chem Res 53(47):18245–18259. https://doi.org/10.1021/ie5025326
Nan J, Han D, Zuo X (2005) Recovery of metal values from spent lithium-ion batteries with chemical deposition and solvent extraction. J Power Sources 152:278–284
Chen X, Kang D, Cao L, Li J, Zhou T, Ma H (2019) Separation and recovery of valuable metals from spent lithium ion batteries: simultaneous recovery of Li and Co in a single step. Sep Purif Technol 210:690–697. https://doi.org/10.1016/j.seppur.2018.08.072
Sun L, Qiu K (2012) Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag 32(8):1575–1582
Chen X, Fan B, Xu L, Zhou T, Kong J (2016) An atom-economic process for the recovery of high value-added metals from spent lithium-ion batteries. J Clean Prod 112:3562–3570. https://doi.org/10.1016/j.jclepro.2015.10.132
Li Q, Fung KY, Xu L, Wibowo C, Ng KM (2019) Process synthesis: selective recovery of lithium from lithium ion battery cathode materials. Ind Eng Chem Res 58(8):3118–3130. https://doi.org/10.1021/acs.iecr.8b04899
Li Q, Fung KY, Ng KM (2019) Separation of Ni, Co, and Mn from spent LiNi0.5Mn0.3Co0.2O2 cathode materials by ammonia dissolution. ACS Sustain Chem Eng (Revision submitted)
Wu HB, Pang H, Lou XW (2013) Facile synthesis of mesoporous Ni0.3Co2.7O4 hierarchical structures for high-performance supercapacitors. Energy Environ Sci 6(12):3619–3626. https://doi.org/10.1039/c3ee42101e
Edwards HGM, Hardman PH (1992) A vibrational spectroscopic study of cobalt (II) oxalate dihydrate and the dipotassium bisoxalatocobalt (II) complex. J Mol Struct 273:73–84
Bickley RI, Edwards HGM, Rose SJ (1991) A Raman spectroscopic study of nickel(II) oxalate dihydrate, NiC2O4 2H2O and dipotassium bisoxalatonickel(II) hexahydrate, K2Ni(C2O4)26H2O. J Mol Struct 243(3):341–350. https://doi.org/10.1016/0022-2860(91)87048-M
Wang D, Belharouak I, Zhou G, Amine K (2013) Synthesis of lithium and manganese-rich cathode materials via an oxalate co-precipitation method. J Electrochem Soc 160(5):A3108–A3112
Mancilla N, Caliva V, D’Antonio MC, Gonzalez-Baro AC, Baran EJ (2009) Vibrational spectroscopic investigation of the hydrates of manganese (II) oxalate. J Raman Spectrosc 40(8):915–920
Donkova B, Mehandjiev D (2004) Mechanism of decomposition of manganese(II) oxalate dihydrate and manganese(II) oxalate trihydrate. Thermochim Acta 421(1):141–149. https://doi.org/10.1016/j.tca.2004.04.001
Gao X, Dollimore D (1993) The thermal decomposition of oxalates: part 26. A kinetic study of the thermal decomposition of manganese(II) oxalate dihydrate. Thermochim Acta 215:47–63. https://doi.org/10.1016/0040-6031(93)80081-K
Allen JA, Scaife DE (1954) The thermal decomposition of nickel oxalate. J Phys Chem 58(8):667–671. https://doi.org/10.1021/j150518a017
Mansour SAA (1994) Spectrothermal studies on the decomposition course of cobalt oxysalts Part III. Cobalt oxalate dihydrate. Mater Chem Phys 36(3):324–331. https://doi.org/10.1016/0254-0584(94)90049-3
Puigdomenech I (2016) Chemical equilibrium diagrams. https://www.kth.se/che/medusa/downloads-1.386254. Accessed 22 Feb 2018
Swain B (2018) Cost effective recovery of lithium from lithium ion battery by reverse osmosis and precipitation: a perspective. J Chem Technol Biotechnol 93(2):311–319
Miller MJ, Scheithauer RA (1990) Method for separation of cobalt from nickel. Google Patents
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Q., Fung, K.Y. & Ng, K.M. Hydrometallurgy process for the recovery of valuable metals from LiNi0.8Co0.15Al0.05O2 cathode materials. SN Appl. Sci. 1, 690 (2019). https://doi.org/10.1007/s42452-019-0705-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0705-z