Abstract
Carbazole—containing motifs are of most desired materials because of its wide range of applications due to π- extended systems, molecular and optical properties was easily tunable via diverse structural modifications. Herein, we reported an efficient and facile polymerization of N-vinyl carbazole (NVK) using multi-site phase transfer catalyst with and without ultrasound condition. The rate of polymerization (Rp) was effectively improved with ultrasound in short time on compare with silent condition. Role of different parameters such as variation of frequency, monomer, initiator, catalyst and temperature, solvents, aqueous and pH on the rate of polymerization of NVK was explored under silent and ultrasound condition (45 kHz/550 W). Activation energy of polymerization was supported for an enhancement of rate under ultrasound condition. From the experimental results, an appropriate kinetic model and role of various parameters in polymerization reaction was discussed. The obtained poly (N-vinyl carbazole) was confirmed and characterized by FT-IR, 1H NMR, TGA and XRD techniques.
Similar content being viewed by others
1 Introduction
Carbazoles are classical class of nitrogen- based heterocyclic, possessing of two benzene rings in central pyrrole motif. Carbazole based polymeric materials have attracted much interest in scientific and industrial arena [1,2,3] and it was considered one of the best desired materials for versatile applications [4, 5] due to facile π—extended systems, easily tunable structures and their natural features of hole-transporting, high charge-carrier mobility and electroluminescent properties [1, 6]. The capability of hole-transporting properties of carbazole was useful in organic electronics applications [7]. Among various carbazole based polymers, a great attention was placed for poly (N-vinyl carbazole) (PNVK) because of easy synthesis and good solubility in common organic solvents [8]. PNVK was a class of promising photoconductive materials used in photocopiers, laser printers, printing plates, and electro-photographic microfilming [9].
Polymerization of N-vinyl carbazole (NVK) was investigated extensively using different methods, for instance, free radical [10], cationic polymerization [11], anionic polymerization [12], nitroxide-mediated polymerization (NMP) [13,14,15], atom transfer radical polymerization (ATRP) [16,17,18,19,20], reversible addition-fragmentation chain transfer (RAFT) polymerization [21,22,23,24,25,26], charge transfer [27], electrochemical [28] and solid state polymerization [29, 30], single electron transfer-living radical polymerization (SET-LRP) [31], organo-hetero atom mediated living radical polymerization [32], and organometallic-mediated radical polymerization [33] etc. Ultrasound condition was used in various organic and polymerization reactions to improve the rate of reaction, facilitates the reactions at ambient conditions etc. [34,35,36,37,38,39,40]. In recent years, ultrasound coupled with phase transfer catalyzed radical polymerization of N-vinyl imidazole [41] and different alkyl methacrylates such as methyl methacrylate [42], ethyl methacrylate [43], glycidyl methacrylate [44] were reported. Consequently, we have also reported ultrasound aided single-site phase transfer catalyzed polymerization of methyl methacrylate [45] and acrylonitrile [46]. In spite of the various methods are reported for the polymerization of N-vinyl carbazole, to the best of our knowledge, the polymerization of N-vinyl carbazole by multi-site phase transfer catalyst and ultrasound conditions has not yet reported. This motivated us to work on polymerization of N-vinyl carbazole (NVK) using 1,4-bis (triethyl methyl ammonium) benzene dibromide (TEMABDB) as multi-site phase transfer catalyst (MPTC) and potassium peroxydisulphate (PDS) as water soluble initiator at 40 ± 2 °C in two phase system under unstirred nitrogen atmosphere with silent and ultrasound condition (45 kHz/550 W). Ultrasound condition facile the polymerization process effectively with two-fold enhancement of yield at short-duration on compare with silent condition. Influence of various experimental parameters on the rate of polymerization was ascertained in both conditions and suitable mechanism was proposed.
2 Experimental
2.1 Materials and solvents
The starting materials 9-Vinylcarbazole (98% of pure, NVK) and initiator, potassium peroxydisulphate (99% of pure, PDS) was purchased from Sigma Aldrich, India used without further purification. The multi-site phase transfer catalyst, 1, 4-bis (triethyl methyl ammonium) benzene dibromide (TEMABDB) was synthesized by adopting the reported procedure as shown in the Scheme 1 [47]. The other solvents are extra-pure grade was used as received from Sigma-Aldrich, Avra and Finar, India. The ultra-pure water was used to make an aqueous phase.
2.2 Instruments
The ultrasound generator having a thermostatic bath equipped with dual frequency (25 and 45 kHz and power of 550 W; Elma Ultrasonicator with dual frequency, Germany) was used for the polymerization (Shown in Fig. 1). 1HNMR spectra of poly (N-vinylcarbazole) were recorded with BRUCKER 400 MHz spectrometer using CDCl3 as solvent and tetramethylsilance (TMS) as an internal reference. FT-IR spectrum of polymer was recorded in (JASCO) the spectral range from 4000 to 400 cm−1. The thermal analysis of poly (N-vinylcarbazole) was carried out by using Inkarp Instrument. The sample weight 3.012 mg was loaded in alumina pans and ramped at heating rate 20 °C per minutes from ambient to 700 °C in nitrogen atmosphere. The powder XRD pattern of the polymer was analyzed using Rigaku X-ray diffractometer.
Polymerization process setup: 1. Polymerization reaction tube consists of equal volume of aqueous and organic phase 2. De-aeration of reaction mixture. 3. Dual frequency Ultrasonicator equipped with thermostatic bath (25 and 45 kHz). 4. Differences in the rate of precipitations of PNVK under ultrasound (right) and silent (left) condition
2.3 Polymerization of N-vinyl carbazole (NVK)
The polymerization reactions were carried out in Pyrex glass tubes provided with inlets and outlets for nitrogen. The monomer (NVK) was dissolved in cyclohexane was the organic phase. The catalyst, ionic and acid strength of phase was adjusted by adding known reagents in the aqueous phase. An equal volume of reaction mixture was thoroughly de-aerated for 20 min. The ultrasound equipment was set in a constant temperature (40 ± 1 °C) with ultrasound condition of 45 kHz; 550 W. The known concentration of initiator was added to reaction tube, which was placed in ultrasonicator, polymerization was started and poly (N-vinylcarbazole) (PNVK) precipitated continuously during polymerization (Scheme 2). After a stipulated time period, the reactions were stopped by pouring the reaction mixture into ice-cold methanol. The polymer was re-precipitated using high pure water and it was filtered through a sintered glass crucible with repeated washing with pure water then dried using vacuum pump after that kept in desiccators to get a constant weight at room temperature. The kinetics of polymerization was examined by tuning the various parameters such as frequency, monomer, initiator, catalyst, solvents, pH and temperature by adopting the aforementioned procedure (Fig. 1). The rate of polymerization (Rp) was determined gravimetrically from the weight of the polymer formed. Rp was calculated from the weight of polymer obtained [45, 48]. The average yield of the polymer was lies between 70 and 75% for each variation of study.
2.4 Mechanistic path ways of polymerization
In two phase reactions, the rate of reaction was attributed to interaction and diffusion between phases. The reaction between immiscible phases was dead slow because of poor collision and diffusion. An easy way to solve such difficulty is to perform the reaction at high temperature and using a co-solvent. However, such conditions are also creates side reactions and complexity in choosing co-solvent. Generally, this kind of situation in two phase system, the rate of reaction is effectively improved by the use of phase transfer catalyst (PTC). PTC capable of extract and transfer ionic reactants of aqueous and or solid phase into organic phase, where the reaction will take place.
On the basis of experimental observations, the mechanistic path ways of polymerization of N-vinyl carbazole in the presence of multi-site phase transfer catalyst under ultrasound condition was proposed. A simple ion exchange reaction between QX (quaternary ammonium salts, multi-site phase transfer catalyst) and KY (Initiator) was occurring to produce QY (quaternary ammonium peroxydisulphate complex) in the aqueous phase. The formed intermediate QY decomposes at the interface in the presence of heat and or ultrasound condition to yield radical anion. Thus generated radical anion was reacting with monomer (M) to form primary monomer radical and polymerization goes up by the successive addition of monomers as shown in Scheme 3 [46]. We observed that the polymerization reactions did not occur in presence of ultrasound alone and also absence of PTC, which proves the role of catalyst in the polymerization.
Role of Catalyst: The polymerization reaction was performed in the absence of catalyst under ultrasound and silent condition ~ 30 min. The effective mixing/movement of two phase was take place with slight changes (turbid), then the reaction mixture was poured into methanol it was disappeared. This observation could be a confirmation of role of catalyst in the polymerization reaction.
3 Results and discussion
N-vinyl carbazole was polymerized in heterogeneous media by multi-site phase transfer catalyst using conventional water soluble initiator. The polymerization of NVK was carried out at 40 ± 2 °C under the silent and ultrasound condition (45 kHz/550 W). To validate the polymerization kinetics, role of different factors such as frequency, monomer, initiator, catalyst and temperature, solvents, aqueous and pH variation on the rate of polymerization of N-vinyl carbazole was explored and its significance was discussed.
3.1 Rely of ultrasound power on polymerization
At the beginning, we have examined the dependence of ultrasound condition on the rate of polymerization of NVK- TEMABDB-PDS system with different ultrasound frequency of 0, 25 and 45 kHz with giving constant power of 550 W and results are presented in Table 1. The rate of polymerization (Rp) was progressively increases with frequency. The high conversion was obtained at the 45 kHz/550 W and it was fixed to evaluate various experimental parameters. Phase transfer catalyzed polymerization rate was doubled with use of ultrasound condition [42,43,44,45,46] and the enhancement of rate in the polymerization is due to chemical and physical effects of ultrasound [49].
3.2 Rely of time on the rate of polymerization (Rp)
The dependence of time on the rate of polymerization of N-vinylcarbazole was examined at different intervals of time by keeping other parameters are constant. The plot of Rp versus time showed the established trends as reported in various phase transfer catalyzed polymerization reactions [47, 48, 50]. The steady state rate of polymerization of NVK was arrived at 2 h and 60 min for silent and ultrasound condition respectively. Further, it was observed the rate of polymerization increases with short duration of reaction time in the presence of ultrasound on compare with silent condition (Fig. 2). This may be due to the effect of ultrasound which could improve the mixing of two phase effectively and making like homogenous phase or improve the formation of radicals [41,42,43,44,45,46].
3.3 Rely of [NVK] on the rate of polymerization (Rp)
The effect of N-vinylcarbazole concentration on the rate of polymerization (Rp) was studied in the range from 0.10 to 0.35 mol/L at fixed other variables under ultrasound and silent condition. It was found that an increasing the concentration of monomer the rate increases for both conditions. Such a routine trends was reported for the phase transfer catalyzed polymerization of different alkyl methacrylates. However, the rate was doubled under ultrasound with short reaction time on compare with silent condition. A plot of 6 + log Rp versus 3 + log [NVK] with the slope was found to be 1.25 and 0.938 for ultrasound and silent condition respectively. The order of unity with respect to NVK was confirmed from the straight line passing through origin in the plot of Rp versus [NVK] (Fig. 3a, b). The order of unity with respect to monomer concentration was well documented in the polymerization of different vinyl monomers using multi-site PTC [45, 46, 51, 52].
3.4 Rely of [PDS] on the rate of polymerization (Rp)
The effect of initiator concentrations in the range of 1.5–2.5 mol/L was explored on the rate of polymerization at fixed all parameters with ultrasound and silent condition. The Rp was doubled with ultrasound condition on compare with silent method. It could be explained by the different radical formation mechanisms. In conventional radical polymerization, radicals are generated by thermal decomposition of initiator where as an ultrasound condition the formation of radicals are effectively improved by various routes like initiator dissociation or water molecule scission, direct exciting monomer or polymer chain etc. [42,43,44]. The reaction order with respect to initiator concentrations of silent and ultrasound condition was obtained from plot of 6 + log Rp versus 3 + log [PDS] was found to be 0.77 and 1.14 for both systems. The plot of Rp versus [PDS] was linear passing through the origin supporting the above deduction (Fig. 4a, b). The higher initiator order can be explained by gel effect or diffusion controlled termination constant [50]. In addition, higher order of initiator concentration suggests that the termination is bimolecular and monomer induced decomposition of PDS was absent. The greater than half order with respect to initiator concentration was reported in phase transfer catalyzed polymerization process [48, 51].
3.5 Rely of [TEMABDB] on the rate of polymerization (Rp)
The role of multi-site phase transfer catalyst (TEMABDB) was varied from 1.5 to 2.5 mol/L to examine its effect on the polymerization rate at fixed other parameters for both conditions. From the observations, it was clear that as rate increases with rise of concentrations and two-fold excess of yield was obtained with ultrasound condition. It may due to presence of ultrasound was promote the mixing of phase, formation of more radicals and make ease the transport of ions between the phases. The slope of linear plot was obtained by plotting of 6 + log Rp versus 3 + log [TEMABDB], the order with respect to [TEMABDB] was found to be 1.07 and 1.00 for ultrasound and silent condition (Fig. 5a, b). The observed order was confirmed from the straight line passing through the origin in a plot of Rp versus [TEMABDB]. The polymerization did not occur in the absence of catalyst as well presence of ultrasound condition even after several minutes [43,44,45,46].
3.6 Rely of temperature on the rate of polymerization (Rp)
The effect of temperature on the polymerization of N-vinyl carbazole was investigated by varying the temperature of medium in the range of 40 °C to 55 °C. It was observed the rate of polymerization increase with raise in temperature because of formation more reactive radicals with high temperature as well ultrasound condition. The activation energy (Ea) was obtained from the Arrhenius plot of 6 + log Rp versus 1/T (Fig. 6). The activation energy (Ea) of 14.78 kJ/mol and 20.10 kJ/mol was obtained for ultrasound and silent condition (Table 2). The activation energy of 36.41 kJ/mol was reported for the polymerization of N-vinyl carbazole using Co(II)-I3X molecular sieves [53]. Activation energy value (Ea) of ultrasound condition was a vital evidences to supports the enhancement of rate with short duration on compare with silent condition.
3.7 Rely of aqueous phase on the rate of polymerization (Rp)
Polymerization reactions was conducted with a constant volume of organic phase and varied volumes of aqueous phase (Vw/Vo = 0.5–5.5) at fixed concentrations of all other parameters for both conditions. The variation of aqueous phase was found to exert no significant change in the rate of polymerization (Fig. 7). A similar kind of trends was observed in most of the phase transfer catalyzed radical polymerization using different alkyl methacrylates [45, 48, 50,51,52, 54].
3.8 Rely of water immiscible solvents on the rate of polymerization (Rp)
The effect of varying dielectric constant of the medium on the polymerization rate was explored using different solvents. The influence of various water immiscible solvents (cyclohexanone, ethyl acetate, toluene, cyclohexane and n-hexane) of dielectric constants and obtained Rp value were presented in Fig. 8 for both conditions. A two-fold enhancement in the rate was observed compare with silent condition. The reason for the observed increase may be due to the formation of greater number of initiating free radicals under ultrasound condition and also polarity of the solvents, which facilitates greater transfer of peroxydisulfate ion from aqueous phase to organic phase [45,46,47,48, 50].
3.9 Rely of water miscible solvents on the rate of polymerization (Rp)
An influence of various water miscible alcohols on the rate of polymerization was examined for both conditions at fixed concentrations of other parameters. The addition of water miscible alcohols in the polymerization medium has resulted moderate increase in the rate as shown in Fig. 9. The reason may attribute to formation of alkoxy radicals from added alcohols, which can further initiate the polymerization by formation of free radicals. This process could be accelerated under ultrasound condition.
3.10 Rely of pH on the rate of polymerization (Rp)
The role of pH is an important parameter in the polymerization reaction. The pH may influence the polymerization rate by coagulating the dispersed phase and affecting the initiation step. We have conducted the polymerization reaction at the pH range of 2 to 10 under both conditions (Fig. 10). Initially, an increase in the rate of polymerization thereafter gradual decrease was observed with the pH range of 2–10. This is due to peroxydisulphate initiated polymerization is negatively affected by the gradual addition of pH in the reaction medium. In the present study the polymerization reaction was conducted at pH of 2.0. This behaviour was reported in phase transfer catalyzed polymerization of vinyl monomers [45, 46].
3.11 Mechanism of phase transfer catalyzed radical polymerization of N-vinyl carbazole
Based on the observed experimental results, it was clear that effective polymerization process was accomplished in two phase system with help of multi-site phase transfer catalyst. However, the rate of polymerization was doubled with short duration under ultrasound condition. The mechanistic path ways of multi-site phase transfer catalyzed polymerization of N-vinyl carbazole using water soluble initiator was shown in Scheme 4. At the beginning, formation of QS2O8 was take place in the aqueous phase on reaction between multi-site PTC (QX2) and initiator (K2S2O8). The generated (QS2O8) intermediate was aggregating on the interfacial border, where it was decomposed in the presence of temperature/ultrasound condition. Thus produce radical anion react with monomer to form monomer radicals so polymerization occurs with successive addition of monomers.
Applying general principles of free radical polymerization and steady-state hypothesis to radical species, the rate law for this mechanism can be derived [45, 54] as follows:
3.12 Analysis of poly (N-vinyl carbazole) (PNVK)
3.12.1 1HNMR spectra of poly (N-vinyl carbazole)
The 1HNMR spectrum of poly (N-vinyl carbazole) was shown in Fig. 11. 1HNMR (400 MHz, CDCl3, δ): a methylene proton gives the signals between 1.3 to 2.0 ppm and N–CH- protons appear at 3.8 ppm. Aromatic protons are well positioned in the aromatic region of 7.0–7.5 ppm.
The vinyl protons of the monomer did not appear at 5–6 ppm in the polymer spectra. It reveals that polymerization reaction was effectively take place at the vinyl position of the monomer.
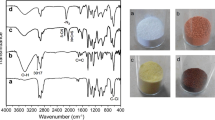
3.12.2 FT-IR analysis of poly (N-vinyl carbazole)
FT-IR spectra of PNVK showed the absence of characteristics stretching band of vinyl group at 852 and 957 cm−1 (Fig. 12) thus confirms that the polymerization takes place selectively through the vinyl groups. The following bands are observed at 2954 and 3054 cm−1 due to aliphatic stretching of –CH and –CH2 groups. The stretching bands at the range of 1213–1157 cm−1 was belongs to out of plane deformation of vinylidene group. In addition to that, a series of bands appeared at 1448 and 1584 cm−1 which are attributed to -CH of carbazole ring, –C=C– stretching of aromatic vinylidene groups. A peak at 1337 cm−1 due to C–N bonds and a band at 3054 cm−1 ascribed to the =C–H stretching vibrations of the aromatic ring [55].
3.12.3 TGA of poly (N-vinyl carbazole)
The TG curve of PNVK revealed that the very minute weight loss at 205° C and loss of weight about less than ten percent weight loss of polymer. The first clear slight weight loss was observed at 227–400 °C with loss of 29.5%. In the second phase polymer showed a weight loss of about 56.5% in the temperature range 401–510 °C. The maximum rate of weight loss appeared around 490° C. In the third stage PNVK showed a weight loss about 9.0% in the temperature range 511–573 °C and appears due to extensive degradation of the polymer, leaving residue of few percent of the sample weight (Fig. 13). This trend was easily associated with reported TG curve of polymer [56].
3.12.4 XRD patterns of poly (N-vinyl carbazole)
The powder X-ray diffraction (XRD) pattern of PNVK was shown in Fig. 14. The poly (N-vinyl carbazole) had a sharp diffraction peak at 2θ = 9.22° and the polymeric chain distance were found at around 21.50 Å. The broad and diffuse hallow peaks at 2θ of 18 to 25° of PNVK confirm the amorphous nature of polymer. A similar type of XRD pattern of PNVK was reported under different reaction conditions [57].
4 Conclusions
In summary, the polymerization of N-vinyl carbazole in two phase system using multi-site phase transfer catalyst and water soluble initiator was successfully accomplished. Indeed, the polymerization rate was effectively doubled with ultrasound on compare with silent polymerization. The various experimental parameters on the polymerization rate were explored and evaluated for both conditions. Rate of polymerization was found to increase with rise of concentration of different variables. Ea value (activation energy) of polymerization was supported an enhancement of rate under ultrasound condition. The obtained polymer was confirmed and analyzed by various techniques. This investigation exhibits the effective role of ultrasound condition on phase transfer catalyzed polymerization. The combined approach of ultrasound and phase transfer catalyst in the polymerization of various N-vinyl monomers may open up new way in the polymerization reactions.
References
Grazulevicius JV, Strohriegl P, Pielichowski J, Pielichowski K (2003) Carbazole-containing polymers: synthesis, properties and applications. Prog Polym Sci 28:1297–1353
Strohriegl P, Grazulevicius JV (1996) Handbook of organic conductive molecules and polymers, 4th edn. Wiley, New York
Mann G (1986) Encyclopedia of polymer science and engineering, 2nd edn. Wiley-Interscience, New York
Li J, Grimsdale AC (2010) Carbazole-based polymers for organic photovoltaic devices. Chem Soc Rev 39(7):2399–2410
Morin JF, Leclerc M (2002) 2, 7-Carbazole-based conjugated polymers for blue, green, and red light emission. Macromolecules 35:8413–8417
Simionescu CI, Grigoras M (1991) Macromolecular donor-acceptor complexes. Prog Polym Sci 16(6):907–976
Liu S, Shi J, Eric W, Steven FM, Blomquist Dave Chiu (2009) Polymer charge-transfer complexes for opto-electronic applications. Syn Met 159:1438–1442
Penwell RC, Ganguly BN, Smith TW (1978) Pol(N-vinyl carbazole): A selective review of its polymerization, structure, properties and electrical characteristics. J Polym Sci: Macromolecular Reviews 13:63–160
Kock Yee L (1993) Organic photoconductive materials: recent trends and developments. Chem Rev 93:449–486
Baethge H, Butz S, Schmidt-Naake G (1997) Living free radical copolymerization of styrene and N-vinylcarbazole. Macromol Rapid Commun 18:911–916
Bilbao E, Rodriguez M, Leon LM (1983) Cationic polymerization of N-vinylcarbazole initiated by trityl salts in nitrobenzene. Polym Bull 10:483–486
Itaru N (2006) Anionic polymerization of N-vinylcarbazole with alkyl lithium as an initiator. Macromolecules 39:6017–6024
Hawker CJ, Bosman AW, Harth E (2001) New polymer synthesis by nitroxide mediated living radical polymerizations. Chem Rev 101(12):3661–3688
Grubbs RB (2011) Nitroxide-mediated radical polymerization: limitations and versatility. Polym Rev 51(2):104–137
Nowakowska M, Zapotoczny S, Karewicz A (2001) Polymeric photosensitizers. Part 4. Synthesis of poly (sodium styrenesulfonate-Block-N-vinylcarbazole) by nitroxide-mediated free radical polymerization. Polym 42(5):1817–1823
Matyjaszewski K, Xia J (2001) Atom transfer radical polymerization. Chem Rev 101(9):2921–2990
Wang TL, Yang CH, Shieh YT, Yeh AC (2009) Synthesis of CdSe-poly(N-vinylcarbazole) nanocomposite by atom transfer radical polymerization for potential optoelectronic applications. Macrommol Rapid Commun 30:1679–1683
Hua J, Chen D, Yu YL (2002) Preparation of C60 bonded poly(N-vinylcarbazole) with C60Cln/CuCl/Bpy catalyst system. Polym Bull 48(2):135–141
Hua J, Chen D, Jing X, Xu L, Yu Y, Zhang Y (2003) Preparation and photo conducting property of C60Clnm-bonded poly(N-vinylcarbazole) with C60Cln/CuCl/Bpy catalyst system. J Appl Polym Sci 87:606–609
Brar AS, Kaur S (2006) Atom transfer radical polymerization of N-vinyl carbazole: optimization to characterization. J Polym Sci Part A 44(5):1745–1757
Kazuhiro N, Mori H (2013) Recent progress in controlled radical polymerization of N-vinyl monomers. Euro Polym J 49:2808–2838
Mori H, Ookuma H, Nakano S, Endo T (2006) Xanthate-mediated controlled radical polymerization of N-vinylcarbazole. Macromol Chem Phys 207:1005–1017
Mori H, Nakano S, Endo T (2005) Controlled synthesis of poly(N-ethyl-3-vinylcarbazole) and block copolymers via RAFT polymerization. Macromolecules 38(20):8192–8201
Perrier S, Takolpuckdee P (2005) Macromolecular design via reversible addition-fragmentation chain transfer (RAFT)/xanthates (MADIX) polymerization. J Polym Sci A 43(22):5347–5393
Favier Charreyre MT (2006) Experimental requirements for an efficient control of free-radical polymerizations via the reversible addition-fragmentation chain transfer (RAFT) process. Macromol Rapid Commun 27(9):653–692
Neelamegan H, Raghavachari D (2011) Controlled polymerization of carbazole-based vinyl and methacrylate monomers at ambient temperature: a comparative study through ATRP, SET, and SET-RAFT polymerizations. J Polym Sci A 49:1021–1032
Natsuume T, Akana Y, Tanabe K, Fujimatsu M, Shimizu M, Shirota Y, Hirata H, Kusabayashi S, Mikawa H (1969) Mechanism of charge-transfer polymerizations: polymerization of N-vinyl carbazole with tetrachloro-p-benzoquinone in benzene. J Chem Soc D 189
Bhadani SN (1991) Electrochemical polymerization of N-vinyl carbazole. J Appl Polym Sci 42:1271–1273
Hazra DK, Chatterjee R (2013) In situ solid state polymerization and characterization of poly (N-vinyl carbazole) encapsulated keggin type polyoxometalate nanocomposite. J Mol Str 1045:139–144
Matsuda T, Higashimura T, Ojsamura S (1968) Solid-state polymerization of N-vinyl carbazole by redox catalyst. J Macromol Sci Chem A 2(1):43–52
Rosen M, Percec V (2009) Single-electron transfer and single-electron transfer degenerative chain transfer living radical polymerization. Chem Rev 109(11):5069–5119
Yamago S (2009) Precision polymer synthesis by degenerative transfer controlled/living radical polymerization using organotellurium, organostibine, and organobismuthine chain transfer agents. Chem Rev 109(11):5051–5068
Hurtgen M, Detrembleur C, Jerome C, Debuigne A (2011) Insight into organometallic-mediated radical polymerization. Polym Rev 51(2):188–213
Mason TJ (1997) Ultrasound in synthetic organic chemistry. Chem Soc Rev 26:443–451
Adewuyi YG (2001) Sonochemistry: environmental science and engineering applications. Ind Eng Chem Res 40:4681–4715
Wang ML, Rajendran V (2007) Kinetics for dichlorocyclopropanation of 1,7-octadiene under the influence of ultrasound assisted phase-transfer catalysis conditions. J Mol Catal A: Chem 273:5–13
Wang ML, Rajendran V (2006) A kinetic study of thioether synthesis under influence of ultrasound assisted phase-transfer catalysis conditions. J Mol Catal A: Chem 244:237–243
Bhanvase BA, Sonawane SH (2014) Ultrasound assisted in situ emulsion polymerization for polymer nanocomposite: a review. Chem Eng Process 85:86–107
Korkut I, Bayramoglu M (2014) Various aspects of ultrasound assisted emulsion polymerization process. Ultrason Sonochem 21:1592–1599
Vivekanandam TS, Gopalan A, Vasudevan T, Umapathy S (1999) Polymerization of acrylamide in the presence of peroxodisulfate and ultrasound. Polymer 40:807–811
Loganathan S, Rajendran V (2013) Ultrasound assisted polymerization of N-vinyl imidazole under phase-transfer catalysis condition—a kinetic study. Ultrason Sonochem 20:308–313
Murugan E, Thamizarasu G (2012) Synthesis and characterization of new soluble multisite phase transfer catalysts and their catalysis in free radical polymerization of methyl methacrylate aided by ultrasound—a kinetic study. J Appl Polym Sci 125:263–273
Sankar K, Rajendran V (2013) Polymerization of ethyl methacrylate under the influence of ultrasound assisted a new multi-site phase-transfer catalyst system—a kinetic study. Ultrason Sonochem 20:329–337
Sankar K, Rajendran V (2012) Ultrasound assisted free radical polymerization of glycidyl methacrylate by a new disite phase-transfer catalyst system: a kinetic study. Ultrason Sonochem 19:1205–1212
Elumalai M, Vajjiravel M (2017) Influence of ultrasonic condition on phase transfer catalyzed radical polymerization of methyl methacrylate in two phase system—a kinetic study. Ultrason Sonochem 38:560–569
Elumalai M, Vajjiravel M (2017) Ultrasonic condition boosts up the rate of phase transfer catalyzed polymerization of acrylonitrile in two-phase system. Appl Petrochem Res 7:85–96
Murugesan V, Marimuthu E, Yoganand KS, Umapathy MJ (2017) Multi-site phase transfer catalyzed radical polymerization of methyl methacrylate in mixed aqueous–organic medium: a kinetic study. Int J Ind Chem 8:241–251
Vajjiravel M, Umapathy MJ (2008) Multi-site phase transfer catalyst assisted radical polymerization of glycidyl methacrylate using potassium peroxydisulphate as initiator—a kinetic study. J Polym Res 15:235–240
Ashokkumar M (2016) Handbook of ultrasonics and sonochemistry. Springer, Singapore
Murugesan V, Marimuthu E (2018) Comparative investigation on radical polymerization of methyl and ethyl methacrylate under multi-site phase transfer catalytic conditions. Appl Petrochem Res 8:1–11
Vajjiravel M, Umapathy MJ (2009) Kinetics and mechanism of multi-site phase transfer catalyzed radical polymerization of ethyl methacrylate. Int J Polymeric Mater 58:61–76
Vajjiravel M, Umapathy MJ (2010) Synthesis, characterization and application of a multi-site phase transfer catalyst in radical polymerization of n-butyl methacrylate—a kinetic study. Int J Polymeric Mater 59:647–662
Biswas M, Banerjee M, Maiti MM (1985) Polymerization of N-vinyl carbazole over cobalt (II) exchanged 13X molecular sieves. Polymer 26(4):625–628
Vajjiravel M, Umapathy MJ (2008) Free radical polymerization of methyl methacrylate initiated by multi-site phase transfer catalyst- a kinetic study. Colloid Polym Sci 286(6–7):729–738
Lo SS, Yang L, Chiu CP (2015) ZnO/poly(N-vinyl carbazole) coaxial nanocables for white-light emissions. J Mater Chem C 3:686–692
Ling QD, Cai QJ, Kang ET, Neoh KG, Zhu FR, Huang W (2004) Monochromatic light-emitting copolymers of N-vinyl carbazole and Eu-complexed 4-vinylbenzoate and their single layer high luminance PLED. J Mater Chem 14:2741–2748
Wang C, Chen Y, Zhang B, Liu S, Chen Q, Cao Y, Sun S (2016) High-efficiency bulk heterojunction memory devices fabricated using organometallic halide perovskite: poly(N-vinyl carbazole) blend active layers. Dalton Trans 45:484–488
Acknowledgements
The authors (VM) acknowledge the Science and Engineering Research Board (DST-SERB), New Delhi for the support under the start-up research Grant No.SB/FT/CS-008/2014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Marimuthu, E., Murugesan, V. Influence of ultrasound on multi-site phase transfer catalyzed polymerization of N-vinyl carbazole in two phase system. SN Appl. Sci. 1, 638 (2019). https://doi.org/10.1007/s42452-019-0661-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0661-7