Abstract
Next-generation magnetic devices will be planar, smaller, light weight, and will do well beyond the present state-of-the-art devices. For this to become a reality, advances in ferrite materials must first be realized. Due to superior coercivity and lower material and processing cost, magnetoplumbites are preferred over the other types of hard ferrites. Magnetoplumbites are important hexagonal ferrites with formula RFe12O19 where R may be barium (Ba), strontium (Sr) or lead (Pb). Energy losses are important issues in these magnetic materials. Although SrFe12O19 have many advantages, but to lower the energy losses we need to improve the resistivity by reducing eddy current losses. Sr-Ba nanoferrites have potential for next generation of energy-efficient magnetic devices. Gadolinium (Gd) belongs to the lanthanide series. In this work, Sr hexaferrites and Sr-Ba hexaferrites doped with rare earth metal like Gd were synthesized to increase their resistivity. Strontium barium hexaferrites with composition Sr1−xBaxFe12−yGdyO19 (x = 0.0, 0.25 and y = 0.0, 0.20) were synthesized by without water and surfactants (WOWS) sol–gel method and co-precipitation method, respectively. All the synthesized samples showed hexagonal structure with P63/mmc space group. Crystallite sizes obtained using Scherrer’s formula were in nanoregime. DC electrical properties as a function of temperature and dielectric spectroscopy as a function of frequency were evaluated and are correlated with the structural properties. A comparative study of different proportions of iron oxide and Gd dopant was carried out, and composition with x = 0.25 and y = 0.20 seems to be a suitable combination for high-frequency efficient devices.
Similar content being viewed by others
1 Introduction
Ferrites are believed to be the widest applied materials as metal oxides. Ferrites have two main kinds, soft magnetic and hard magnetic ferrites. Soft ferrites are prepared by elementary materials which are iron oxide and other metal oxides like manganese oxide, nickel oxide, cobalt oxide and zinc oxide [1, 2]. Hard ferrites are widely used as permanent magnetic materials which are fabricated using strontium, barium and lead in oxide or nitrate form [3, 4]. Hexagonal ferrites have varied application as permanent magnets due to truncated cost, high resistivity, striking magnetic and electrical properties [5]. Characteristics of nanomaterials are influenced by surface atoms when their size approaches nanoscale. M-type hexaferrites are highly resistive complexes having iron oxide as main constituent. Among different types of ferrites, the hexagonal ferrites have numerous applications and a vast period of production. Also, these have high coercive force, high Curie temperature and excellent chemical stability [2]. The M-type hexaferrites usually have hexagonal symmetry of space group of P63/mmc [3]. The structural and electrical behaviors of ferrites are based on factors like crystallite size, particle size, chemical composition and synthesis procedures. M-type is important class of hexagonal ferrites with formula RFe12O19 where R may be strontium (Sr), barium (Ba) and lead (Pb) [6]. Hexagonal ferrites have electrical and magnetic properties which are dependent on the condition in which synthesis takes place and the site occupation of substitute cation with different Fe3+ sublattices named as trigonal bipyramid (2b), tetrahedral (4f) and octahedral (12 k, 2a and 4f2) of hexagonal structure [6]. To enhance the properties of Sr hexaferrite and make the material useful for different applications, various scientists doped different elements and combination of various metallic cations.
Strontium and barium have reasonable magnetic properties. Barium ferrite is important for several reasons like stable crystalline features; it has high coercive force and low density and consists of ultra-fine particle with high specific magnetic energy [6]. Significance of barium strontium hexaferrites is due to holding chemically stability, high coercivity and high magnetic anisotropy. Both strontium and barium have same crystalline structure as that of magnetoplumbite. A lot of work has been done to understand the impact of various dopants for iron and barium. The consequence of frequencies on the electrical behavior can be elucidated based on charge transport phenomena [7]. The usual fabrication methods used for hexaferrites are hydrothermal techniques, co-precipitation approach and simplified or WOWS sol–gel method [8, 9]. WOWS sol–gel is wet chemical method (a simplified and more economic form of sol–gel method) used in the fields of material science and ceramics engineering. This is a preferable method due to simplicity, high-purity goods and can produce dense coatings to overcome corrosion. In divergence to others, co-precipitation method has gained deep attention on immense industrial production, low cost and allows to control the particle size more resourcefully [8].
Electrical properties (AC and DC) were measured at room temperature as well as with temperature using LCR meter over frequency range from 20 Hz to 3 MHz. Crystallinity, crystallite sizes and phase purity were obtained by X-ray diffraction. The main effort of this paper is to provide current information about electrical and structural properties of Sr-Ba hexaferrites.
2 Experimental details
The precursor chemicals used in the synthesis of samples were Sr(NO3)2, Fe(NO3)3·9H2O, GdN3O9·6H2O and Ba(NO3)2. All the reagents were of analytical grade and were used without any further treatment. The Sr hexaferrite samples substituted with Ba and Gd with nominal composition Sr1−xBaxFe12−yGdyO19 (x = 0.0, 0.25 and y = 0.0, 0.20) were prepared by two different chemical methods. The precursors were prepared by dissolving Sr(NO3)2, GdN3O9·6H2O and Fe(NO3)3·9H2O in deionized water for co-precipitation method. Molarity for all the solutions was maintained at 0.4 M, and they were separately stirred for 20 min. After mixing the precursor solutions at room temperature, the temperature of mixture was raised to 70 °C at the rate of 10° per minute. Sodium hydroxide solution (2.0 M) was prepared by dissolving it in deionized water. This was required to control the pH (= 12) of the solution. The mixture of all was kept for constant magnetic stirring at 70 °C for 45 min. Brown precipitates were obtained after heating and stirring. The precipitates were washed by deionized water and then dried in an oven at 105 °C for overnight. For preparation of hexaferrites by WOWS sol–gel, ethylene glycol was used as a gelling agent. Precursor reagents mass-to-ethylene glycol ratio was optimized at 1:14 [8, 9]. The solutions thus obtained were heated at 80 °C till the gel is formed, and then, temperature was raised to 150 °C to get the fine powder. All prepared powder samples were calcined at 910 °C for 20 min. Calcined powders were pressed into pellets of diameter 13 mm and were sintered at 920 °C for 20 min.
3 Results and discussion
3.1 X-ray diffraction analysis
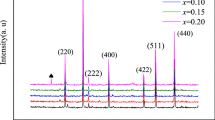
X-ray diffraction (XRD) is the useful method for obtaining the insight of structure. Figure 1 shows the XRD pattern of all the samples. The hexaferrites formation with space group P63/mmc is confirmed. Maximum intensity peaks were observed (ICDD # 01-080-1197, 00-048-0156 and 00-002-0919) with Miller Index (107) for all the compositions except for the composition (x = 0.25, y = 0.20), for which Miller Index was (114). The crystallite size is calculated using Scherrer’s formula.
Comparison of various structural parameters is given in Table 1. All the samples were with hexagonal structure as primary structure with no sign of any secondary phases. The effect of dopant was also observed by the peak shift toward the higher values of 2θ angle. The observed variation in both the lattice parameters was due to the difference in ionic radii of Sr2+ (1.32 Å); Ba2+ (1.49 Å); Fe3+ (0.79 Å); and rare earth Gd3+ (1.07 Å) ions. The doping of bigger size ions caused a slight shift in the X-ray diffraction peaks and expanded lattice which is governed by Vegard’s law [10, 11]. Rare earth Gd3+ ions are expected to replace Fe3+ sites due to comparable ionic radii and same valence states.
3.2 Electrical properties
AC electrical measurements were taken by LCR meter in frequency range 20 Hz–3 MHz at room temperature, and the DC electrical properties were evaluated as a function of temperature.
3.3 Dielectric constant
Performance of dielectric constant at room temperature is given in Fig. 2. The dielectric behavior can be explained based on Maxwell–Wagner two-layer model [10, 11]. It is observed in Fig. 2 that the dielectric constant for all the samples decreased with the increase in frequency due to loss of interfacial polarization. The dielectric constant decreased with increase in frequency of the applied field. The behavior reflects normal behavior of polar dielectric materials, where a polar dielectric material which contributes to the polarization lags the applied field at higher frequencies. The variation of dielectric constant with frequency shows the dispersion in studied samples is Maxwell–Wagner type and is in well agreement with Koop’s phenomenological theory of dielectrics [12, 13]. According to these models, a dielectric structure is supposed to be composed of well-conducting grains, separated by poorly conducting grain boundaries. The grain boundaries are formed during sintering process due to superficial reduction or oxidation of crystallites in porous materials due to their direct contact with sintering atmosphere. The polarizations decrease with increasing frequency and then reach a constant value beyond a certain frequency limit where electron hopping cannot follow the changes in an alternating field [12,13,14]. The dielectric constant of prepared samples can be calculated from usual relations [15].
3.4 Dielectric loss tangent
Figure 3 shows the dielectric loss tangent which is decreasing with the increase in frequency. The energy loss at higher frequency is given by loss tangent (tanδ). This usually appears because of lag in change in dipole orientation with respect to the applied electric field. This may be due to defects and the presence of impurities in the material along with the reasons mentioned in the previous section.
3.5 AC conductivity
The variation of AC conductivity of hexaferrites as a function of frequency is shown in Fig. 4. AC conductivity is determined by Eq. 2,
where εo is the permittivity of free space, ω is the angular frequency, έ is the dielectric constant and tanδ is the dielectric loss. It is observed from graph that σac of ferrites increased with the increase in frequency. It is seen that the AC conductivity increases with the increase in frequency of an applied field. Since increase in frequency enhances hopping frequency of charge carriers between Fe2+ and Fe3+ at the octahedral site, the conductivity increases [13, 16]. The sample (x = 0.25 and y = 0.00) showed high conductivity due to dipole polarization. With enhanced frequency hopping of electrons also increased which heightened the conductivity. The increase in conductivity does not mean that the number of charge carriers increases, but only the rate of hopping of charge between the charge carriers, Fe2+ and Fe3+, increases. The rate of hopping is enhanced once the frequency of the applied field increases, resulting in increase in conductivity and decrease in resistivity [17]. For compositional effect, it can be shown from Fig. 4 that the value of conductivity decreased with the increase in the concentration of Ba2+ and Gd3+. This is attributed to the greater jump lengths due to higher ionic radii of Ba2+ as compared to that of Sr2+ in the first case and to the reduced charge carriers due to replaced Fe2+/Fe3+ ions by Gd3+ in the latter case. Other than compositions, crystallite sizes are also affecting the properties and are responsible for slightly anomalous response [13, 15].
3.6 Impedance
Spectra of impedance and real and imaginary parts of impedance were determined at room temperature and are shown in Fig. 5. The frequency range used was 20 Hz–3 MHz. Following Eqs. (3, 4) are used to compute the real and imaginary part of impedance.
where Z is impedance and ‘θ’ is phase angle. From Fig. 5, it can be observed that real part of impedance (Z/) decreased with growing frequency for all samples and reaches a constant value later. This indicates the mobility of polarons. There are three generic relaxation dispersion situations, which may occur for solids while discussing Z/ as a function of frequency: conductive system associated charge carriers, dielectric dispersion usually arising from dipole rotation of lattice entities and the presence of both types of dispersion within measured frequency range. Three different dipolar relaxation processes and an intrinsic conductivity contribution may be involved in this case. Imaginary part of the impedance (Fig. 5) showed a decreasing trend for the all cases. In ferrites, conduction mechanism is due to intra-grain conduction and inter-grain boundaries. The occurrence of relaxation peaks in the imaginary part of complex impedance at low frequencies may be due to the existence of the space–charge relaxation at low frequencies, which is related to the charge carriers in association with the oxygen vacancies. The conductivity is dominated by the short-range hopping of charge carriers [18,19,20].
Figure 6 shows the Cole–Cole plot (complex impedance plot) for the impedance of all the samples at room temperature. Similar trends could be seen in the literature [8, 14]. The impedance spectroscopy helps in the separation of grain and grain boundary because each of them has different relaxation times, resulting in separate semicircles in complex impedance plane. The spectrum reveals relatively large grain boundary contribution to the total resistivity because close to the grain boundaries, transport properties of the materials are controlled by imperfections, expected to be present in higher concentration than in grains, leading to an additional contribution to the inter-grain (grain boundary) impedance. The internal space charge created at the grain boundaries may lead to a significant increase in the concentration of mobile effects [18, 19].
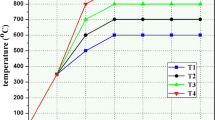
3.7 Temperature-dependent electrical properties
The samples were subjected to temperature increase, and the conduction behavior was observed (Fig. 7). For DC conductivities, it was observed that significant increase was observed after a certain threshold step. This could be attributed to availability of charge carriers and ease in hopping between the sites.
4 Conclusion
Strontium barium hexaferrites with composition Sr1−xBaxFe12−yGdyO19 (x = 0.0, 0.25 and y = 0.0, 0.20) were synthesized by WOWS sol–gel method and co-precipitation method. XRD pattern indicates the single-phase hexagonal structure of prepared sample. Crystallite sizes were found to be in range from 55 to 86 nm. In the frequency range of 20 Hz–3 MHz, dielectric constant has higher values at lower frequencies. Dielectric loss tangent decreased due to decrease in Fe2+ ion concentration which is the normal behavior of dielectric properties of hexaferrites. Increase in the concentration of barium as well as that of Gd increased the resistivity and stability of the material. Increased resistivity with doping will be helpful for energy-efficient devices due to reduced eddy current losses. At higher frequencies, AC conductivity increased sharply due to increased hopping rate of ions, relaxation and re-orientation of ions. These properties make hexaferrites suitable for potential application in electronic devices to prevent energy losses. Variation in electrical properties is quite anomalous with composition and frequencies, and this indicates that these materials could be used in a wide variety of applications as per the suitability.
References
Mahmood SH, Awadallah A, Maswadeh Y, Bsoul I (2015) Structural and magnetic properties of Cu-V substituted M-type barium hexaferrites. Mater Sci Eng 92:012008
Bhatia PG (2016) A study of structural and physical properties of substituted barium hexaferrites. IJCPS 5:28–33
Rusianto T, Wildan MW, Abraha K, Kusmono (2015) The magnetic and mechanical properties of sintered ceramic magnets SrxBa1−xFe12O19. IJET 15:41–45
Cernea M, Negrea RF, Ciuchi IV, Baldisserri C, Trusca R, Galassi C (2016) Dielectric characterization of BaxSr1−xFe12O19 (x = 0.05–0.35) ceramics. Ceram Int 42:1050–1056
Dawarl N, Chitkara M, Sandhu IS, Jolly JS, Malhotra S (2016) Structural, magnetic and dielectric properties of pure and nickel-doped barium nanohexaferrites synthesized using chemical co-precipitation technique. Cogent Phys 3:1208450
Bakis Y, Auwal IA, Unal B, Baykal A (2016) Maxwell–Wagner relaxation in grain boundary of BaBixLaxFe12−3xO19 (0.0 < x < 0.33) hexaferrites. Compos B 99:248–256
Wu Q, Yu Z, Hao H, Chu Y, Xie H (2017) The effect of pH value on strontium hexaferrites: microstructure and magnetic properties. J Mater Sci 28:12768–12775
Nasir S, Asghar G, Malik MA, Anis-ur-Rehman M (2011) Structural, dielectric and electrical properties of zinc doped nickel nanoferrites prepared by simplified sol–gel method. J Sol-gel Sci Tech 59:111–116
Nasir S, Anis-ur-Rehman M, Malik MA (2011) Structural and dielectric properties of Cr-doped Ni–Zn nanoferrites. Phys Scr 83:025602
Iqbal MJ, Ashiq MN, Hernandez-Gomez P, Munoz JM (2008) Synthesis, physical, magnetic and electric properties of Al-Ga substituted co-precipitated nanocrystalline strontium hexaferrite. J Magn Magn Mater 320:881–886
Zhong W, Ding W, Zhang N, Hong J, Yan Q, Du Y (1997) Key step in synthesis of ultrafine BaFe12O19 by sol–gel technique. J Mater Sci 168:196–202
Batoo KM, Mir FA, El-Sadek MSA, Shahabuddin M, Ahmed N (2013) Extraordinary high dielectric constant, electrical and magnetic properties of ferrite nanoparticles at room temperature. J Nanopart Res 15:2067
Batoo KM, El-Sadek MSA (2013) Electrical and magnetic transport properties of Ni-Cu-Mg ferrite nanoparticles prepared by sol-gel method. J Alloys Compd 566:112–119
Batoo KM, Kumar G, Yang Y, Al-Douri Y, Singh M, Jotania RB, Imran A (2017) Structural, morphological and electrical properties of Cd2+ doped MgFe2-xO4 ferrite nanoparticles. J Alloys Compd 726:179–186
Asghar G, Anis-ur-Rehman M (2012) Structural, dielectric and magnetic properties of Cr–Zn doped strontium hexa-ferrites for high frequency applications. J Alloy Compd 526:85–90
Batoo KM (2011) Microstructural and Mössbauer properties of low temperature synthesized Ni-Cd-Al ferrite nanoparticles. Nanoscale Res Lett 6:499
Batoo KM, Ansari MS (2011) Influence of doping on Dc conductivity of Mn ferrites. In: AIP conference proceedings, vol 1349, no 1, pp 1021–1022
Batoo KM, Kumar S, Lee CG (2009) Study of ac impedance spectroscopy of Al doped MnFe2−2xAl2xO4. J Alloys Compd 480:596–602
Batoo KM, Kumar S, Lee CG (2009) Finite size effect and influence of temperature on electrical properties of nanocrystalline Ni-Cd ferrites. Curr Appl Phys 9:1072–1078
Batoo KM, Kumar S, Lee CG (2009) Influence of Al doping on electrical properties of Ni-Cd nano ferrites. Curr Appl Phys 9:826–832
Acknowledgements
Higher Education Commission (HEC) is acknowledged for providing the financial support during this research. Assistance of Ms. Uzma Afsar and Ms. Nahall Niazi is also thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anis-ur-Rehman, M. Topical advances in synthesis for energy-efficient magnetoplumbite nanoferrites. SN Appl. Sci. 1, 636 (2019). https://doi.org/10.1007/s42452-019-0652-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0652-8