Abstract
In developing countries like Pakistan, there is a lack of implementation of the environmental laws to manage discharges of untreated effluents, loaded with heavy metals, into freshwater. To evaluate the potential human health impacts from exposure to such polluted water, this study assessed the concentration of six heavy metals (i.e., Zn, Fe, Pb, Cu, Cd, and Mn) in the surface water in lower Sindh, Pakistan. Water samples were collected from eight locations around Kotri barrage at Indus River both during pre-monsoon and monsoon seasons to determine the temporal variations of the concentration of heavy metals. The concentrations of heavy metals were then compared with the drinking water quality guidelines of the World Health Organization (WHO). It was observed that in most cases, the concentrations of metals were significantly higher compared to WHO guidelines during the pre-monsoon period. To determine the potential human health risk associated with the exposure of heavy metals, both oral hazard index (HIoral) and dermal hazard index (HIdermal) were estimated. The results indicated that during pre-monsoon, HIoral was higher than the threshold limits at every location, while HIdermal was higher only at three locations. Nonetheless, during monsoon, these indices were within limits. It suggested that the health of the people dependent on these freshwater streams in the study area is at considerable risk. Finally, the findings of this study recommended the enforcement of the laws in its true spirit to regulate effluent discharges into these streams in order to avoid health vulnerability of millions of people.
Similar content being viewed by others
1 Introduction
Heavy metal pollution in freshwater has become a global environmental problem because of toxicity and potential risks to the ecosystem and human health [1,2,3]. To address this issue, the United Nation’s Sustainable Development Goal 6 (SDG 6) clearly states that by 2030, the quality of water should be improved through reducing the effluents and its untreated discharge, and increasing the recycling and reusing of wastewater. Currently, the situation of surface water quality in Pakistan is not better than other developing Asian nations, and Pakistan is striving to achieve SDGs.
Lack of restrictions on disposal of untreated wastewater into freshwater supply, non-compliance of pollutions standards and misuse of the water resources are very common in Pakistan and it is worsening the quality of freshwater. The situation is even worst in urban areas where sewage water is dumped into freshwater canals, and in several places, the water is not suitable for consumption [4]. A study conducted by Ullah et al. [5] in Peshawar city of Pakistan revealed that river water gets contaminated through urban wastewater dumping. Industrialization and unplanned urbanization with poor sewage services are increasing the negative impacts on the rivers and especially on the streams flowing through the urban areas [6]. The haphazard growth without any pollution restriction and bulk amount of municipal and industrial untreated wastewater either from upstream or locally added into the waterways pose a severe threat to human health and ecosystem [5, 7, 8]. Due to these practices, waterborne diseases are common in Pakistan constituting about 80% of total diseases and 30% of the deaths [9]. Such practice has created a nuisance to the residents of Pakistan especially to the downstream areas. It is evident that under such circumstances, where various sources are contributing contaminants, the overall ecological health is under risk.
River Indus is the main river of Pakistan, which is the primary source of fresh water to all sectors of the country including industry and agriculture. But unfortunately, it is treated as a sink of polluted wastewater. Heavy metals reach water bodies through industrial and urban municipal wastewater discharges, agricultural runoffs, and through atmospheric deposition [10]. These metals are transported from upstream through river and canals systems. Communities living in the lower riparian zone of the river Indus are vulnerable to be exposed from acute to chronic toxicity of heavy metals. These metals also accumulate into the soil and through plants enter into our food system and therefore restricted suburban agriculture was recommended by Jadoon and Malik [11].
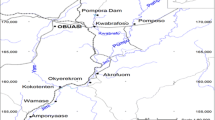
Among six barrages on the river Indus, Kotri is the last one before it finally falls in the Arabian Sea. Four major canals originating from Kotri barrage supply water to downstream users. Due to potential upstream polluted water inflows into the river system as well as locally added effluents, there is a need to ascertain the status of heavy metals in the river Indus at Kotri barrage and the canals originating from this barrage as shown in Fig. 1. It is essential because these canals not only supply water to agricultural fields but also for domestic uses of millions of people living in Hyderabad, Karachi, and several small cities and towns [12].
Among several pollutants, heavy metal concentration in water bodies under such circumstances poses many risks to human health. The residents dependent on such water can be exposed to heavy metals through multiple routes including but not limited to the drinking of metal-contaminated water, consuming foods cultivated with metal-polluted water [13,14,15], and through using that water for other domestic purposes. It has both carcinogenic and non-carcinogenic effects and may damage different human body organs. Considering these facts, this study aims to evaluate the status of heavy metals in water from the river Indus at lower Sindh, Pakistan, and to assess the human health risk associated with these metals.
2 Materials and methods
2.1 Study area and selection of sampling locations
This study was conducted in the southern part of Sindh province of Pakistan, at Kotri Barrage and the canals originating from this barrage as shown in Fig. 1. Among four canals, one canal flows on the western side of Kotri barrage, which is known as Kalri Baghar (KB) feeder. It is the main source of freshwater to Karachi, the largest and the most populous city of Pakistan, and Thatta district for domestic and industrial usage. It also irrigates some agricultural land. The other three canals, including Phuleli, Pinyari, and Akram canals, originate from the eastern side of the Kotri Barrage. These three canals largely supply water to agriculture. Moreover, these canals are the only source of freshwater to the residents of Hyderabad, the second largest city of Sindh and Badin districts.
To ascertain the status of heavy metal contamination in the water near Kotri barrage and in the canals, eight sampling locations were selected. Two sampling locations (SL1 and SL2) were selected at Kalri Baghar feeder at upstream and downstream of the canal, respectively. Similarly, two sampling locations (SL3 and SL4) were selected on the upstream and downstream of Kotri Barrage, respectively, at the Indus River. Since all eastern canals originate from the same point, only one sampling location (SL5) was selected at the upstream of eastern canals, while three downstream locations were selected at Phuleli (SL6), Panyari (SL7), and Akram canals (SL8). To take into account the temporal variations, sampling was performed during monsoon, i.e., during July and August, when water gets diluted due to the rainy season and 4 months after monsoon, i.e., January to February (which is hereafter called as pre-monsoon, as the flow of water in the river Indus remains stable until the following monsoon season).
2.2 Sampling, transportation, and preservation of water samples
American Public Health Standard Association (APHA) [16] procedures were followed for the sampling, transportation, and preservation of water samples. The samples were collected in 500 mL polyethylene bottles soaked overnight in 0.1% hydrochloric acid (HCl) and rinsed three times with distilled water. To ensure representative water quality, three samples were collected from each location by grabbing sampling from the 0.5 m depth from the surface in bottles. Each bottle was then double zip-locked in polythene plastic bags to avoid any contamination and properly labeled. Samples were immediately placed in an ice box and shifted to the laboratory and refrigerated at 4 °C till analyzed.
2.3 Preparation of samples
The collected water samples (500 mL each) were pre-concentrated by evaporation at 100 °C until the volume was reduced to 50 mL. Samples were then cooled and filtered through a filter paper ‘Whatman No. 42’ to exclude suspended particles before instrumental analysis.
2.4 Analyses for heavy metals detection
Heavy metals (Zn, Fe, Mn, Cu, Pb, and Cd) were analyzed using ‘APHA 311A’ [16] method on a Flame Atomic Absorption Spectrophotometer (AAS) (A1200, Aurora Biomed, Canada) as described by Cesar Lopes Geraldino et al. [17]. The minimum detection limits (MDL) of the metals on the AAS are found to be 0.00032, 0.03, 0.0001, 0.000024, 0.001, and 0.0005 mg per liter (mg L−1) for Zn, Fe, Mn, Cu, Pb, and Cd, respectively. The concentration (mg L−1) of heavy metals detected through AAS was then compared with the drinking water quality guidelines of the World Health Organization [18] .
The quality control and quality assurance were provided by adopting standard operating procedures. This includes the use of high-purity chemicals and reagents and thoroughly cleaning glasswares used in the laboratory analysis with detergent and rinsing it three times with deionized water. For dilutions, only deionized water was used. Standard calibration curves were generated by converting the absorbance observed by AAS into concentrations. To verify the precision and proficiency of the methods adopted in the analysis, recovery and reproducibility studies were undertaken. For this certified standard reference solutions for Mn, Cd, Pb, Zn, Fe, and Cu prepared from BDH Chemicals were used. The percentage recovered from the recovery studies for Mn, Cd, Zn, Fe, Pb, and Cu were 98%, 93%, 99%, 98%, 100%, and 100%, respectively.
2.5 Statistical analyses
One sample t test was used to compare the mean value concentrations of heavy metals with their respective WHO permissible limits. Independent sample t test was used to compare the concentration of metals between monsoon and pre-monsoon seasons. Pearson correlation analysis was performed to determine the relationship between the concentrations of heavy metals. At places where the concentrations of the metals were below the MDL of the AAS, then half of the MDL values were included for the quality assurance of the analysis [19]. The statistical analyses of metal concentrations were performed using SPSS 22, while health risk calculations were conducted using Microsoft Excel. Further representation of the metal concentration and hazard index were also plotted using Microsoft Excel.
2.6 Human health risk assessment
Oral and dermal exposures to heavy metals were considered for the human health risk assessment since oral and dermal are the most significant pathways [20,21,22]. The potential hazards to human health from individual metals were evaluated by Chronic Daily Intake (CDI) and Hazard Quotient (HQ). The CDI through oral ingestion (CDIoral) and dermal absorption (CDIdermal) were calculated by using Eqs. 1 and 2 following the Agency for Toxic Substances and Diseases Registry [23].
where ‘C’ is metals concentrations in water, ‘IR’ is the water intake rate, ‘EF’ is exposure factor, ‘ET’ is exposure time, ‘CF’ is conversion factor, ‘BW’ is the body weight, ‘P’ is the permeability coefficient, and ‘SA’ is the skin surface area.
The average values of IR, EF, ET, CF, BW, P, and SA are in Table 1. The values of the BW’s were calculated from the average weights for the age group from 15 to 66 years for males, 15 to 67 years for females, following the standards of United States Environmental Protection Agency [24], and 0–15 years for children [25]. The values for SA were taken as the average of the males and females in the age group from 15 to 70 years, and for children as the average of the males and females in the age group from 3 to 15 years [23].
To appraise the non-carcinogenic risk from exposure to heavy metals present in water, HQ was used as given by Chai et al. [26], Shah et al. [27], Naz et al. [28], and Karim [29] in Eq. 3. According to this, when the HQ < 1, it reflects that the exposed masses are safe from metals risk.
RfD is the reference dose for oral and dermal exposure routes; derived by USEPA from the dose–response experiments [30]. The values of RfDoral (mg kg−1 d−1) were taken from the existing literature as 0.3, 0.3, 0.02, 0.04, 0.0005, and 0.0014 [31, 32] for Zn, Fe, Mn, Cu, Cd, and Pb, respectively. Similarly, the values of RfDdermal (mg kg−1 d−1) were taken as 0.06, 0.14, 0.00096, 0.012, 0.00042, and 0.000025 [33] for Zn, Fe, Mn, Cu, Pb, and Cd, respectively.
To estimate the collective human health risk caused by various metals, Hazard Index (HI) was calculated by adding up the HQ for every individual metal following Eq. 4 [26, 33,34,35]. The HI values were calculated separately for male, female, and children.
3 Results and discussion
3.1 Heavy metals concentrations
Mean concentrations of Zn, Fe, Mn, Cu, Pb, and Cd at each sampling locations are shown in Table 2. It was observed that the overall concentrations of metals were in the order of Zn > Fe > Mn > Cd > Pb > Cu and Cu > Zn > Fe > Mn > Cd > Pb during monsoon and pre-monsoon seasons, respectively. The observed highest mean concentrations of Zn (6.6 mg L−1), Fe (3.98 mg L−1), Cd (0.2 mg L−1), Mn (0.64 mg L−1), Pb (0.03 mg L−1), and Cu (4.34 mg L−1) at various sampling locations were more likely attributed to the anthropogenic activities including domestic and mainly industrial wastewater discharges, runoffs, and irrigation return flows.
Pre-monsoon values for Pb and Cu were higher than monsoon values. Mn and Fe values were higher in pre-monsoon than monsoon except for Mn at SL5 and SL3 and Fe at SL7 and SL6. Zn showed mixed trend and was higher in pre-monsoon than monsoon only at SL2–SL1. Cd values were observed higher in monsoon than pre-monsoon except at location SL1. Thus, overall, the measured data showed mostly higher metal levels in the pre-monsoon period compared to the monsoon periods regardless of the sampling locations. It might be due to low water levels during the pre-monsoon to raise the overall concentrations of the metals. Similar trends were observed by Saleem et al. [36] that higher rainfall during the monsoon season mixes a considerable volume of non-contaminated water with the contaminated water to reduce total metal concentrations in Lakes in Pakistan. On the contrary, higher metal concentrations observed during the monsoon season at few locations might be due to the mixing of more polluted water with less contaminated water during the rainy season. This might be contributed by the sewage overflows and runoff from agricultural and dumping sites wastes in the vicinity [37].
We observed an elevated concentration of heavy metals in our study as compared to similar studies conducted in lower Sindh. For instance, the mean concentration of Fe in Jamshoro was observed by Baig et al. [38] as 0.19 mg L−1, which is lower than the findings of this study. Similarly, the mean concentration of Pb in surface water samples in Karachi were observed higher than the WHO permissible limits [39] which confirm the findings of this research too. Moreover, the mean concentration of Cd in the Malir River, Karachi, was found 0.04 mg L−1 by Waseem et al. [40], which is considerably lower compared to the findings of this study. Soomoro et al. [7] found that during winter season, the heavy metal concentration at different locations of Phuleli canal is 0.32 mg L−1, 1.72 mg L−1, 0.182 mg L−1, 0.20 mg L−1, 0.0032 mg L−1, and 0.038 mg L−1 for Zn, Fe, Cu, Mn, Cd, and Pb, respectively. This comparison indicates that generally, over time, the concentration of heavy metals is increasing in the surface water in lower Sindh which is somehow alarming.
From downstream Kotri barrage, all three eastern canals flow through Hyderabad city, while Kalri Baghar feeder flows through Kotri industrial area. A significant amount of contaminated wastewater is discharged into these canals without any proper or even with no treatments, and therefore differences among upstream and downstream concentrations were observed. It is very difficult to classify the individual sources of the metal concentrations in all canals. Nevertheless, anthropogenic activities are largely responsible for these metal concentrations in eastern canals, while several industries of Kotri Industrial area are responsible for polluting the western canal (Kalri Baghar Feeder). In addition to this, agricultural waste also reaches these canals through seepage and runoff during monsoon.
The mean values of heavy metals were compared with its respective WHO permissible limits using one sample t test and it was observed that the concentration of Cd, Fe, and Pb is significantly higher than the permissible limit. Additionally, the results of independent sample t test show significant differences between monsoon and pre-monsoon metal concentrations except for Zn as shown in Table 3.
3.2 Health risk assessment
Considering the most critical impact pathways of heavy metals on human health, i.e., ingestion and absorbed through the skin, HIoral and HIdermal from exposure to water contaminated with heavy metals were calculated for adult men, women, and children separately as shown in Table 4. The HI results associated with the heavy metal contaminations in water during monsoon period show that HIoral for men, women, and children was below the threshold limit (i.e., HIoral < 1), at the entire eight sampling locations. However, in the pre-monsoon season, the values of HIoral for men, women, and children were considerably higher and exceeded the threshold limit at all the sampling locations. Among HIoral, the maximum oral values for men, women, and children were observed as 14, 16.5, and 16.7, respectively. Higher values of HIoral in pre-monsoon season for all the three groups of the population especially women and children are dependent on the stream water. It indicates that a potentially significant health impact on those people is directly dependent on this water.
Dermal Hazard Index (HIdermal) values, for men, women, and children, during both monsoon and pre-monsoon seasons, were lower than the threshold limit (i.e., HIdermal < 1) at every location except at locations SL1, SL2, and SL4, where it was observed higher than the permissible limits for pre-monsoon season. Higher values of HIdermal at these locations suggest more susceptibility to skin ailments to the inhabitants living around the study area, and most importantly children are more vulnerable.
This variation in results between monsoon and pre-monsoon is due to the difference in water flow rate during monsoon and pre-monsoon seasons. During monsoon, the flow rate of water is high; thus, it reduces the concentration of heavy metals per unit volume of water, while in pre-monsoon, the flow of water is shallow which does not attenuate the concentration heavy metals. The HIoral and HIdermal indices above the threshold limit in pre-monsoon suggest a considerably high industrial discharge without adequate treatment. Figure 2 depicts the oral and dermal health hazard indices of male, female, and children during monsoon and pre-monsoon.
Similar studies were conducted in Bangladesh [41] and China [19], where industrial and residential areas have been polluting the surface water sources. In Bangladesh, the assessment of the heavy metal (i.e., Cu, Mn, Ni, and Zn) concentration in Bangshi River water indicates that concentrations in water were above the safe limits. In China, the results of the study conducted in Upper Han River [21] revealed that the concentration of 11 heavy metals varies seasonally and the highest concentrations were observed in the rainy season.
To explore further, contribution analysis of each heavy metal from each sampling location toward the HI during monsoon and pre-monsoon was conducted as shown in Fig. 3. A significant variation in the contribution of each metal toward HI in different sampling locations and seasons was observed. The most critical contributors during monsoon toward HIoral were Mn, Zn, and Fe and toward HIdermal, Mn, and Zn were contributing maximum. On the other side, during pre-monsoon, the HIoral was mostly influenced by Cu and Cd, and a smaller contribution from Pb and Mn was observed. Similarly, Cu, Cd, and Mn were found as the primary contributor toward HIdermal during pre-monsoon.
Pearson correlation analysis was performed to know the relationship among metals concentration during monsoon and pre-monsoon seasons which is shown in Table 5. During pre-monsoon season, a significant positive correlation was observed between Zn and Cd (0.545), Fe and Cd (0.599), and Fe and Zn (0.925), and low significant positive correlation was found between Pb and Cd (0.359). However, during the monsoon season, significant positive correlation was witnessed between Cd and Mn (0.423), and low significant positive correlation was found between Pb and Fe (0.359), and significant negative correlations were established between Zn and Mn (− 0.651) and Zn and Cd (− 0.464), indicating different metals entering the canals after flooding from various sources. Though it is challenging to identify the primary source of these metals, significant correlations showed that metals with high correlation coefficient are coming from similar sources and these metals concentrations fluctuate together.
4 Conclusion and recommendations
This study revealed that most of the metals exhibited significant spatial and seasonal variability during the study period. When the concentrations of the metals were compared with WHO permissible limits, most of those reflected relatively higher values, particularly during the pre-monsoon (dry) season, with few exceptions. It was also observed that comparatively higher metal concentrations were detected near the urban areas or adjacent to the entry of main streams. While assessing the non-carcinogenic health risk, it was revealed that the hazard indices were above the threshold limit during pre-monsoon while within the limit during monsoon season. Variations in the metal concentrations among different sampling locations indicate that urban sewage water in the surrounding areas is contributing significantly toward the concentrations of metals. Higher chances of non-carcinogenic health risks were exhibited by heavy metals in children, male, and female at eight sampling locations in pre-monsoon in the order of SL1 > SL2 > SL4 > SL6 > SL8 > SL3 > SL5 > SL7, while in monsoon, there was no chance of health risk from exposure to water in the study area. This study recommends that there should be strict implementation of the regulations to restrict the unchecked disposal of industrial and domestic effluents into the freshwater to preclude health damages to society. This study further recommends conducting similar research at a larger scale considering the impacts of downstream industries and urban setting as well as health risk assessment for these potentially carcinogenic metals.
References
Chowdhury S, Mazumder MAJ, Al-Attas O, Husain T (2016) Heavy metals in drinking water: occurrences, implications, and future needs in developing countries. Sci Total Environ 569–570:476–488. https://doi.org/10.1016/j.scitotenv.2016.06.166
Ali MM, Ali ML, Islam MS, Rahman MZ (2016) Preliminary assessment of heavy metals in water and sediment of Karnaphuli River, Bangladesh. Environ Nanotechnol Monit Manag 5:27–35
Rehman IU, Ishaq M, Ali L et al (2018) Enrichment, spatial distribution of potential ecological and human health risk assessment via toxic metals in soil and surface water ingestion in the vicinity of Sewakht mines, district Chitral, Northern Pakistan. Ecotoxicol Environ Saf 154:127–136
Azizullah A, Khattak MNK, Richter P, Häder D-P (2011) Water pollution in Pakistan and its impact on public health—a review. Environ Int 37:479–497. https://doi.org/10.1016/j.envint.2010.10.007
Ullah Z, Khan H, Waseem A et al (2013) Water quality assessment of the River Kabul at Peshawar, Pakistan: industrial and urban wastewater impacts. J Water Chem Technol 35:170–176. https://doi.org/10.3103/s1063455x1304005x
Qadir A, Malik RN, Husain SZ (2008) Spatio-temporal variations in water quality of Nullah Aik-tributary of the river Chenab, Pakistan. Environ Monit Assess 140:43–59. https://doi.org/10.1007/s10661-007-9846-4
Soomro A, Siyal AA, Mirjat MS, Sial NB (2014) Seasonal variations of trace elements and heavy metal concentrations in Phuleli Canal water (Sindh), Pakistan. Sarhad J Agric 30:73–82
Mitra S, Sarkar SK, Raja P et al (2018) Dissolved trace elements in Hooghly (Ganges) River Estuary, India: risk assessment and implications for management. Mar Pollut Bull 133:402–414
Daud MK, Nafees M, Ali S et al (2017) Drinking water quality status and contamination in Pakistan. Biomed Res Int 2017:1–18. https://doi.org/10.1155/2017/7908183
Reza R, Singh G (2010) Heavy metal contamination and its indexing approach for river water. Int J Environ Sci Technol 7:785–792
Jadoon WA, Malik RN (2019) Geochemical approach for heavy metals in suburban agricultural soils of Sialkot, Pakistan. SN Appl Sci 1:1–11. https://doi.org/10.1007/s42452-019-0167-3
Sohag MA, Syed MA (2014) Pollution and encroachment of Phuleli canal along the periphery of Hyderabad city of Pakistan. Am J Biomed Sci 6:72–81
Jiang H, Qin D, Chen Z et al (2016) Heavy metal levels in fish from Heilongjiang river and potential health risk assessment. Bull Environ Contam Toxicol 97:536–542
Antoniadis V, Levizou E, Shaheen SM et al (2017) Trace elements in the soil-plant interface: phytoavailability, translocation, and phytoremediation—a review. Earth Sci Rev 171:621–645
Antoniadis V, Shaheen SM, Boersch J et al (2017) Bioavailability and risk assessment of potentially toxic elements in garden edible vegetables and soils around a highly contaminated former mining area in Germany. J Environ Manag 186:192–200
Rice EW, Baird RB, Eaton AD, Clesceri LS (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association, American Water Works Association, Water Environment Federation
Cesar Lopes Geraldino H, Izabelle Simionato J, Formicoli K, de Souza FT et al (2015) Evaluation of the electrode wear and the residual concentration of iron in a system of electrocoagulation. Desalin Water Treat 57:1–11
WHO (2017) Guidelines for drinking-water quality: forth edition incorporating the first addendum, 4th edn. WHO Library Cataloguing-in-Publication Data Guidelines, Geneva
Wu H, Chen B (2014) Using statistical and probabilistic methods to evaluate health risk assessment: a case study. Toxics 2:291–306. https://doi.org/10.3390/toxics2020291
Wu B, Zhao DY, Jia HY et al (2009) Preliminary risk assessment of trace metal pollution in surface water from Yangtze River in Nanjing section, China. Bull Environ Contam Toxicol 82:410
Li S, Zhang Q (2010) Risk assessment and seasonal variations of dissolved trace elements and heavy metals in the Upper Han River, China. J Hazard Mater 181:1051–1058
Qu L, Huang H, Xia F et al (2018) Risk analysis of heavy metal concentration in surface waters across the rural–urban interface of the Wen-Rui Tang River, China. Environ Pollut 237:639–649
ATSDR (2005) Public health assessment guidance manual. Atlanta, Georgia
USEPA (2011) Exposure factors handbook, 2011th ed. National Technical Information Service, Springfield, VA, Washington DC; EPA/600/R-09/052F
Khan K, Lu Y, Khan H et al (2013) Health risks associated with heavy metals in the drinking water of Swat, northern Pakistan. J Environ Sci 25:2003–2013
Chai L, Wang Z, Wang Y et al (2010) Ingestion risks of metals in groundwater based on TIN model and dose-response assessment—a case study in the Xiangjiang watershed, central-south China. Sci Total Environ 408:3118–3124
Shah MT, Ara J, Muhammad S et al (2012) Health risk assessment via surface water and sub-surface water consumption in the mafic and ultramafic terrain, Mohmand agency, northern Pakistan. J Geochem Explor 118:60–67
Naz A, Mishra BK, Gupta SK (2016) Human health risk assessment of chromium in drinking water: a case study of sukinda chromite mine, Odisha, India. Expo Health 8:253–264
Karim Z (2011) Risk assessment of dissolved trace metals in drinking water of Karachi, Pakistan. Bull Environ Contam Toxicol 86:676–678
USEPA (2018) 2018 Edition of the drinking water standards and health advisories. Off. Water U.S. Environmental Protection Agency, DC 1–20
Chen J, Qian H, Gao Y, Li X (2018) Human health risk assessment of contaminants in drinking water based on triangular fuzzy numbers approach in Yinchuan City, Northwest China. Expo Health 10:155–166. https://doi.org/10.1007/s12403-017-0252-z
Memon AH, Ghanghro AB, Jahangir TM, Lund GM (2016) Arsenic contamination in drinking water of district Jamshoro, Sindh, Pakistan. Biomed Lett 2:31–37
Zheng N, Liu J, Wang Q, Liang Z (2010) Health risk assessment of heavy metal exposure to street dust in the zinc smelting district, Northeast of China. Sci Total Environ 408:726–733
Sarigiannis DA, Hansen U (2012) Considering the cumulative risk of mixtures of chemicals—a challenge for policy makers. Environ Health A Global Access Sci Source 11:1–12
Kumar M, Ramanatahn AL, Tripathi R et al (2017) A study of trace element contamination using multivariate statistical techniques and health risk assessment in groundwater of Chhaprola Industrial Area, Gautam Buddha Nagar, Uttar Pradesh, India. Chemosphere 166:135–145
Saleem M, Iqbal J, Shah MH (2019) Seasonal variations, risk assessment and multivariate analysis of trace metals in the freshwater reservoirs of Pakistan. Chemosphere 216:715–724
Kansal A, Siddiqui NA, Gautam A (2013) Assessment of heavy metals and their interrelationships with some physicochemical parameters in eco-efficient rivers of Himalayan region. Environ Monit Assess 185:2553–2563
Baig JA, Kazi TG, Arain MB et al (2009) Evaluation of arsenic and other physico-chemical parameters of surface and ground water of Jamshoro, Pakistan. J Hazard Mater 166:662–669. https://doi.org/10.1016/j.jhazmat.2008.11.069
Ul-Haq N, Arain MA, Badar N et al (2017) Drinking water: a major source of lead exposure in Karachi, Pakistan. East Mediterr Health J 17:882–886. https://doi.org/10.26719/2011.17.11.882
Waseem A, Arshad J, Iqbal F et al (2014) Pollution status of pakistan: a retrospective review on heavy metal contamination of water, soil, and vegetables. Biomed Res Int 2014:1–29. https://doi.org/10.1155/2014/813206
Saha N, Sa M, Boshir M et al (2017) Industrial metal pollution in water and probabilistic assessment of human health risk. J Environ Manag 185:70–78. https://doi.org/10.1016/j.jenvman.2016.10.023
Acknowledgements
The authors would like to acknowledge the financial and logistic support provided by US-Pakistan centers for advanced studies in water (USPCAS-W), Mehran University of Engineering and Technology (MUET) Jamshoro, Sindh, Pakistan, to conduct this study. The authors would also like to acknowledge three anonymous reviewers, whose valuable comments helped in making substantial improvements in this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Imran, U., Ullah, A., Shaikh, K. et al. Health risk assessment of the exposure of heavy metal contamination in surface water of lower Sindh, Pakistan. SN Appl. Sci. 1, 589 (2019). https://doi.org/10.1007/s42452-019-0594-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0594-1