Abstract
The current strategy reports the recovery of precious group metals (PGMs) from Na2CO3 roast of Saudi Arabian ophiolitic chromite ore at 1160 °C under atmospheric O2. The influence of roasting temperature (960–1200 °C) and leaching agents (H2O, HCl, H2SO4) and their concentrations on the recovery of PGMs was studied. Chromite roast concentrates a reasonable percentage of approximately 45% with an overall 43% Cr2O3. PGMs in the original ore was petitioned and ends up in Na2CrO4 liquor whereas the liquor and a solid roast residues contained the rest of chromite components (Fe2O3, Al2O3, others). Na2CrO4 in the water or H2SO4 leachate was reduced with SO2 gas to give crystalline basic chrome sulfate with excellent yield (> 99%) and purity (Fe < 0.1%). PGMs in the Na2CrO4 liquor were extracted into methylisobutylketone (MIBK) after shaking with ammonium pyrrolidine dithiocarbamate (APDC). In MIBK extract, the PGMs species was then stripped by shaking with HgCl2–HNO3 (1.0 mol L−1). Ir and Pt in the produced PGEs cake were found up to 4.0 and 0.5 µg/g, respectively whereas the majority of Ni (0.5% as NiO) was retained in the solid roast residues. The proposed method offers a simple approach with good cost-effectiveness for PGEs recovery and production of basic chrome sulfate with Fe content less than 0.1%. Leaching with water offers a simple, an eco-friendly and cost-effectiveness approach for the production of a basic chromium sulfate product with high quality yield of PGEs as a byproduct with minimum Fe content (< 0.1% m/m) required for the tanning industry compared to the method reported.
Similar content being viewed by others
1 Introduction
It is well known, chromite mineral is a solid solution of four different spinels form a combination of the following potential end members: FeAl2O4, MgAl2O4, FeCr2O4, MgCr2O4, Fe3O4 and MgFe2O4 spinels [1]. The ophiolitic chromites defined as {(Fe,Mg)(Cr,Al,Fe)2O4} are characterized by different contents and assemblages of PGM’s. Variable PGE contents (0.033–1.88 µg/g) and enrichment in Os, Ir and Ru have been reported from chromite deposits from Mayari-Baracoa ophiolite belt in Eastern Cuba [2]. Ophiolitic chromites from south west of Turkey are enriched in IPGM (Os, Ir, and Ru) over PPGM (Pt, Pd, and Rh) with PGM concentrations between 61 and 1305 µg/kg [3, 4]. Berit chromites in SE Turkey contain Pt (10–1155 µg/kg) and Pd (3–2518 µg/kg) enrichments [5]. The chromites from Middle Arm. Brook (Newfoundland, Canada) are characterized by relatively high total PGEs (up to 1028 µg/g) with enrichment in PPGE relative to IPGE [5]. The Pindos ophiolite complex, north-western Greece hosts various chromites deposits and are characterized by low PGE grades with a few localities in the area of Korydalos (SE Greece) enriched in PGMs +Au (up to 29.3 µg/g) [6].
Bearing the fact that the environment liability on manufacturing is increasing, research must focus on the development of other processing directions, which lighten the delicate of wastes, materials and energy, by dropping the time for handling and falling the waste volume at the end of the process [7]. Saudi Arabia Ophiolitic chromites from Al’ Ays area contain PGM’s with maximum values of 2.570, 0.687, 0.840, 5.800, 6.200, and 3.300 µg/g for Pt, Pd, Rh, Ru, Ir and Os, respectively with an average of total PGM’s (40 samples) about 972 µg/kg and about 1500 µg/g Ni [8, 9]. The continued interest in ophiolitic chromites arises from the fact that the single largest resource of PGMs in the world is the layers of chromite in the Bushveld complex South Africa. The ophiolitic chromitites have been extensively investigated worldwide for the possible occurrence of PGM’s [10]. The enrichment and extraction of PGEs from Bushveld type chromite ore has been reported by magnetic, gravity and flotation technologies (physical separation) [10–15].
Leather tanning industry and the use of PGEs are the important and growing industries in Saudi Arabia (SA) and annually consumes about 3000 tons (imported) of basic chrome sulfate (Tanning and leather goods committee, Jeddah, Chamber of Commerce and Industry, Personal communication). Low grade and low tonnage of Saudi chromite ore and the fine grain size of the platinum group minerals (less than 20 microns) make them not amenable for flotation.
PGMs are vital for the progress of recent manufacturing as well as designing and developing high technology products in our daily lives. Hence, the worldwide demand of PGEs is rising quickly and predicted to surpass the supply by 40,000 tons annually. PGEs (Pt, Pd, Ir, Os, Rh, Ru) are often linked with gold and are of main profitable significance [16–19]. Recently, magnetic solid phase extraction has been used for the separation of PGMs from leach solutions and wastewater [20]. PGMs have very few occurrences where they are present in an ore deposit at economically extractable levels [21, 22]. PGMs are energetic elements for the developing of existing production as well as planning and developing high technology products used in our daily lives. Moreover, as far as the authors are aware, the enrichment and recovery of PGMs from Saudi Arabian chromite ore is of great interest and importance and it has not been attempted. Therefore, a lab scale attempts to use Saudi ophiolitic chromite for recovery PGMs and production and basic chrome sulphate is of great importance in recent years. Hence, the present study is focused on: (1) Enrichment of PGMs and Ni through separation of Cr2O3 which constitutes about 50% of chromite; (2) Recovery of PGM’s (byproduct) by solvent extraction using methylisobutylketone (MIBK) and the complexing agent ammonium pyrrolidine dithiocarbamate (APDC); and finally (3) Developing of efficient procedures for the production of basic chromium sulfate [(Cr2(SO4)2 (OH)2)] (Target component for leather tanning industry) from chromite ore processing residue using low cost, efficient and eco-friendly leaching agent such as water and/or H2SO4.
2 Materials and methods
2.1 Reagents and materials
All chemicals and solvents used were of analytical reagent grade. Low density polyethylene (LDPE) bottles, Nalgene were carefully cleaned first with hot detergent, soaked in 50% HCl (Analar), HNO3 (2.0 mol L−1), subsequently washed with dilute HNO3 (0.5 mol L−1) and finally rinsed with distilled water. LDPE bottles were used for leaching experiments of the chromite ore–Na2CO3 roast with the leaching agents (H2O, HCl, H2SO4). A stock solution (0.1% w/v) of the reagent ammonium pyrolidine dithiocarbamate (Merck, Darmstadt, Germany) was prepared in acetone. A series of standard BDH solutions (Poole, England) solutions (100 µg mL−1) of the PGM’s and nickel were prepared in dilute nitric acid. Saudi Arabian Ophiolitic chromite ore samples were collected from Wadi Al Hawanet and Jabal Al Wasq areas.
2.2 Apparatus
A Perkin Elmer inductively coupled plasma-optical emission spectrometry (Optima 4100 DC Shelton, CT, USA) was operated at the optimum operational parameters for determination of the major elements. A Perkin Elmer ICP-MS Sciex model Elan DRC II (California, CT, USA) was used for analysis of PGMs at the optimized operational parameters. Deionized water was obtained from Milli-Q Plus system (Millipore, Bedford, MA, USA). Digital micropipettes 10–1000 µL (Volac) were used for preparation of more diluted solutions. A pH Meter (Thermo Orion model 720, Fisher Scientific, MA, USA) was employed for pH measurements.
2.3 Experiment #1: determination of chemical composition of chromite ore
Different methods have been used for the dissolution of chromite, e.g. lithium tetraborate flux [23], sodium peroxide fusion and mixtures of HNO3, HF and HClO4 [21], MnO2–LiSO4–sulphuric acid mixtures [24]. In this study, four Saudi Arabian chromite ore samples collected from Wadi Al Hawanet (samples I–III) and Jabal Al Wasq (sample IV) areas were digested in the acid mixture of HClO4–HNO3–HF (1:1:1 v/v) for 3 h [25]. The resultant solutions were analyzed for major and, trace elements, and PGM’s by ICP-OES and/or ICP-MS.
2.4 Recommended experimental procedures
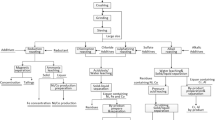
2.4.1 Roasting of the chromite ore with Na2CO3
Antony et al. [26] and Zhang et al. [27] have been processed chromite ore by roasting with sodium carbonate, limestone and dolomite at 1000–1200 °C [27]. In the current study, the traditional method [26] was used for the recovery of chromate based chromite–Na2CO3 roast ore with sodium carbonate has been carried out with some modifications as follows:
-
1.
Four chromite samples (120–180 ± 0.01 g) were roasted with sodium carbonate in clay crucibles at about 1160 °C in air for 2 h. Iron in chromite roast reacts with sodium carbonate to produce NaFeO2 + NaAlSiO4 and other complex sodium-aluminium silicate according to the following reaction [1, 26]:
Hence, the overall reaction can be expressed as follows:
Some other products e.g. NaFeO2 + NaAlSiO4 + other Na–Al silicates may be formed. The solid residue of roasting step was crushed and selectivity leached with water and/or or sulfuric acid until equilibrium. After leaching, the solid residues was quantitatively separated out from the water leachate. The recovery of Chromium(VI) as Na2CrO4 increased in the leachate on increasing the amount of Na2CO3 above the stoichiometric ratio demonstrated in the following equation [26]:
-
2.
The water leachate (liquor) was analyzed for major metal oxides, trace elements and PGMs using ICP-OES and/or ICP-MS. The un reacted chromium oxide remained in the chromite roast with the refractory oxides and ends up in the waste stream for landfills. ICP-OES analysis of the roast reduces after leaching was used to calculate the extraction yield according to the following equation:
The percent yield of sodium chromate was also calculated by ICP-OES analysis of chromium in the leachate after leaching using the equation:
-
3.
After leaching the chromite roast with water, the solid residue was quantitatively separated out and digested with a mixture of concentrated HClO4, HNO3 and HF as reported [25]. The reaction mixture was heated slowly for 1 h at 100–150 °C on a hot plate. After the evolution of NO2 fumes had ceased, the reaction mixture was evaporated almost to dryness. The solid residue was re-dissolved in HNO3 (10.0 mL, 1.0 mol L−1) and the resulting mixture was filtered through a Whatman 41 filter paper, transferred to volumetric flask (25.0 mL) and the solution was completed to the mark with deionized water and finally analyzed for major oxides, trace elements and PGM’s using ICP-OES.
2.4.2 Influence of roasting time of the chromite ore with Na2CO3
In order to study the influence of roasting time of chromite ore with Na2CO3, an accurate weight (120–140 ± 0.01 g) of the chromite ore sample collected from Wadi Al Hwanet area (Sample I) were mixed well in clay crucible with an excess mass of sodium carbonate above the stoichiometric value in Eq. (1). The solid mixtures were roasted in muffle at about 1160 °C in air for various annealing time (2–6 h). The solid residues were left to cool and finally subjected to study the influence of leaching agent and contact time.
2.4.3 Influence of leaching agent and contact time of the chromite roast
The effect of various leaching agents e.g. boiled H2O, HCl (1.0 mol L−1) and H2SO4 (1.0–7.5 mol L−1) and contact time (4–10 h) on the recovery of Cr, Al, Ni, and PGEs was carried out on the chromite roasted at 5 h annealing time. The liquor (leachates) of each leaching agent at various contact time were critically filtered and finally analyzed for trace metal ions including Cr, Al, Fe and Ni oxides by ICP-OEs and ICP-MS for PGMs.
2.4.4 Preparation of basic chrome sulphate
The liquors containing Na2CrO4 obtained in the previous experiments were acidified with sulfuric acid to form Na2Cr2O7 according to the following equation [27]:
Basic chrome sulfate can then be manufactured by simple reduction of the resultant Na2Cr2O7 in acid media to basic chrome sulfate with SO2 according to the following equations:
In a separate conical flask, the produced SO2 gas from the reaction (9) was allowed to react with Na2CrO4 acidified with H2SO4 in another conical flask according to Eq. (10) to form basic chrome sulfate Cr2(SO4)3 (OH)2.
Upon evolution of SO2 gas (Eq. 9), the aqueous acidified solution of Na2CrO4 is reduced in the second flask according to Eq. (10) to give a green and crystalline basic chrome sulfate after slow evaporation.
2.4.5 Extraction and recovery of PGMs
Extraction of the PGMs was performed following the recommended procedures reported earlier as follows: colloidal AuNPs solution was prepared as follows [28, 29]: In the present study, an accurate volume (500.0 mL) of sodium chromate water lecahte of an accurate weight of the chromite roast with Na2CO3 was shaken well with MIBK (15.0 mL) in the presence of APDC (5 mL. 0.5% m/v) for 5 min. After equilibrium and layer separation, the organic phase was separated out. The PGEs were then stripped from the organic extract by shaking with 10 mL of HNO3 (10 mL, 1.0 mol L−1) containing Hg(NO3)2 (0.01% m/v) to form stable chelate with APDC and release other metal ions including PGMs. The PGE’s in the organic phase was then stripped in the nitric acid layer and finally analyzed by ICP-OES and/or ICP-MS.
3 Results and discussion
3.1 Production of basic chromium sulfate
The chemical compositions of the four chromite ore samples collected from Wadi Al-Hwanet area (I, II, III) and Jabal Al Wasq (IV) are summarized in Table 1. The results were found comparable with the analyses of 41 chromite samples from Al’Ays ophiolite complex [28]. The content of Al2O3, Fe2O3, MgO and Cr2O3 oxides were found in the range 7–24%, 13–25%, 9–16%, and 44–68%, respectively in agreement with the data reported [28]. The relatively low level of SiO2 (0.09–2.2%) in the reuse samples points to the low content of gangue silicate minerals which control the sodium chromate yield which it can varies from 20 to 82% [26, 28].
Prichard et al. [28] have analyzed 41 chromite samples from Al’Ays ophiolite complex by Ni–sulfide fire assay method In that work, samples were properly digested with a mixture of HF/HNO3/HCl/HClO4 and analyzed by ICP-MS. The concentration levels of Pt, Pd, Ru, Rh, Ir and Os were in the range 3–2570; 4–6870; 41–5800; 5–840; 13–6200 and from 6 to 3300 µg/g, respectively. The ∑PGE’s values are < 1 µg/g in 24 samples, from 1.0 to 3.0 µg/g in 14 samples, with two samples > 3 µg/g [28]. In the present study, the ∑PGM’s of the studied four samples (I–IV) as analyzed by Gen analysis Laboratory, Australia were found comparable and are in acceptable agreement with the range reported from Al’Ays chromite [8, 28]. In this study, the high content of PGE’s given in Table 1 may be ascribed to the nugget effect [30–33]. The heterogeneous distribution of the PGEs in the chromite samples may also account for this trend.
The percentage of unreacted chromite ore in the Na2CO3 roast after leaching with water varies between 59.81 and 86.63% as demonstrated in Table 2. This behavior is most likely attributed to: (1) The formation of Na2CO3–Na2CrO4 liquid phase which forms a thin film around chromite grains that inhibits diffusion of oxygen to the reaction interface [26] and (2) The large sizes of the crushed roast of small surface area. The percent of reacted chromite ore can be maximized by increasing the roasting time of chromite ore with Na2CO3 and also by raising the annealing temperature up to 1400 °C [26]. Antony et al. [26] have reported that, maximum percent formation and leaching of sodium chromate generally did not exceed 80–82%.
The results of chemical analysis of major oxides (wt%), trace elements and PGEs (µg mL−1) of the water leach liquors of chromite roast are summarized in Table 3. The percent yield of Na2CrO4 in Table 3 is quite low as compared to the work of Antony et al. [26]. Thus, it can be concluded that, the roast residue in the present study can be considered as a lean ore and it may require further roasting time and high annealing temperature with Na2CO3 to convert most of the chromium oxide content in the chromite ore to sodium chromate [26]. In the roast, Na2CO3 reacts with Cr2O3 in the spinels forming sodium chromite (Na2Cr2O4) as an intermediate phase which in turn is further oxidized in air producing sodium chromate (Na2CrO4). The grade content (%) of Cr2O3 of the chromite–Na2CO3 residue could further be improved by multiple stage consecutive preconcentration techniques, multi-gravity separator and high-intensity induced-roll magnetic separator [34].
In batch mode of separation, the effect of leaching time and temperature of the roasted chromite -Na2CO3 at different annealing ((roasting) time (2 to ≈ 6 h) on the extent of chromium and iron recovery as Cr2O3 and Fe2O3 was further conducted. In this experiments, a series of leaching agent’s such as H2O, HCl (1.0 mol L−1), and H2SO4 (1.0 mol L−1) were used. The influence of roasting time (2–6 h) on the extraction of Cr and Fe as Cr2O3 and Fe2O3 were critically investigated. After cooling, the chromite roast were shaken with the leaching agents (water, HCl, and H2SO4) for various time intervals and the resultant Cr and Fe in the leachates were analyzed by ICP-OES. The results for the extraction of Cr2O3 and Fe2O3 are shown in Figs. 1 and 2, respectively. The percentage yield of leached Cr2O3 (%) increased in the following order (Fig. 1): HCl > H2SO4 > H2O whereas the leaching percentage of Fe2O3 followed the order (Fig. 2): H2SO4 > HCl > H2O at room temperature. The leaching efficiency of Cr2O3 (%) using HCl (% Conv. Up to 39%) and H2SO4 (%Conv. Up to 36%) at concentration of 1.0 mol L−1 is greater than with H2O (% Conv. Up to 26%) (Fig. 1).
The influence of H2SO4 and HCl concentrations (1.0–7.5 mol L−1) individually on the leaching efficiency of Al2O3, MgO, Fe2O3 and Cr2O3 was carried out. Representative results using H2SO4 at various concentrations are shown in Fig. 3. The data revealed that, liquors produced by leaching with HCl and H2SO4 at different concentrations (0.5–7.5 mol L−1) contain higher Cr2O3 and Fe2O3 compared with liquor produced by leaching with H2O (Figs. 1 and 2).
The influence of contact time (4–10 h) on the leaching efficiency of Cr2O3 with water was critically carried out. The liquors were separated out and finally analyzed for chromium by ICP-OES. The leaching efficiency of Cr2O3 with water increases on increasing the contact time up to a maximum value after 8 h and leveled off at longer time as demonstrated in Fig. 4. The maximum value of tolerable Fe in the marketable basic chrome sulfate is 0.1%, whereas the present study provided leaching with water as a simple approach with cost-effectiveness for the production of high quality with good yield of basic chrome sulfate. Minimum iron content less than 0.1% required for the leather tanning industry was achieved in the present study. The proposed study is also eco-friendly for the production of basic chrome sulfate and it gives a product nearly free from iron content when compared to the published methods. Thus, leaching with water is highly recommended for the production of basic chrome sulfate.
3.2 Production and extraction of PGM’s
The composition of the water leach liquors of chromite roast (Table 3) reveals that, almost pure Na2CrO4 solution was achieved and most of the trace elements of the ore remained in the roast residue. The majority of the Ni content of the ore ends up in the chromite roast residue with values up to 0.4% NiO. The PGE’s content of the ore was preconcentrated in the liquor because most of the PGEs form highly water soluble sodium salts e.g. sodium palatinate and some other sodium or potassium salts of PGEs [35]. The amount of PGEs remaining in the solid roast residues (minerals and alloys) are most likely enclosed within the un reacted chromite grains as demonstrated in Table 2.
The Na2CrO4 liquors obtained from roasting chromite ore with sodium carbonate was further acidified with H2SO4 to convert Na2CrO4 into Na2Cr2O7 according to Eq. (8) and subsequently reduced with SO2 gas prepared from reaction of Na2SO3 with dilute HCl (Eq. 9) to give basic chrome sulfate (Target compound in tanning industry) according to Eq. (10). The proposed method for basic chrome sulfate appears to be suitable for routine production of basic chrome sulfate. In conclusion, the proposed procedure could be utilized readily for routine production of the salt since the conventional methods in literature survey suffer from drawbacks e.g. high cost, multiple steps and are time consuming while the proposed method offers a simple system which is cost- effective, requires short analytical time coupled with good reproducibility, and nearly free from iron oxide content compared to the reported methods [26]. Na2SO4 is a part of the end product and it is not separated and remains as an inert diluent. The developed extraction method provided better separation, low cost and easy production of high grade basic chromium sulfate with less iron content as compared with the published methods [26].
3.3 Recovery of PGM’s from chromite roast
Solvent extraction has a wide and well defined use in separation and/or preconcentration of PGMs from ores and solutions [36, 37]. The PGM’s were enriched from the water liquor of sodium chromate by solvent extraction using APDC as a chelating agent and methylisobutyl ketone as extractant solvent. The fact that, the complexing agent APDC able to form stable chelates with PGMs according to the following equation:
where M = PGMs and M(APDC)m]org n+ is the precious metal complex in the organic solvent. The resultant PGMs were then stripped from the organic solvent by shaking with nitic acid solution contains mercuric ions. The results of the chemical analysis after solvent extraction and recovery of PGMs, Co, V and Ni are given in Table 4. These revealed better separation compared to other solvent extraction [38] and precipitation steps and precipitation methodology [39, 40]. The low level of the PGM’s in the liquor is most likely attributed to the incomplete dissolution of the chromite ore, since; the un-converted ratio of chromite varies between 30 and 87% (m/m). The Na2O content of the residue varies between 4.0 and 10.0% (Table 2). The high content of Na2O in the residue is most likely attributed to the presence of excess Na2CO3 and/or inefficient leaching of Na2CrO4.
4 Conclusion and future perspectives
The present study provides a useful set of data on the production of basic chromium sulphate from chromite ore. The percent yield of sodium chromate is strongly dependent on the roasting time, particle size of roast, surface area, contact and shaking time, concentration and nature of leaching agent. Chromite dissolution during roasting with Na2CO3 s is variable and the percentage of the un reacted chromite lies in the range 30–87%. The remaining residue of the roast after leaching is rich in chromite which is considered as a lean ore and can be roasted again with Na2CO3 for further recovery of sodium chromate. The incomplete recovery of chromium is most likely attributed to formation of a binary Na2CO3–Na2CrO4 liquid during the roasting process which covers the chromite grains and prevents the diffusion of oxygen and subsequently chromite dissolution. Basic chromium sulphate which is used in tanning industry is produced from the sodium chromate liquor after acidification with H2SO4 and reaction with SO2. PGE’s were collected in Na2CrO4 liquor while the majority of Ni (0.5% as NiO) was retained in the roast residue. Leaching with water offers a simple, an eco-friendly and cost-effectiveness approach for the production of a basic chromium sulfate product with high quality yield with minimum iron content (< 0.1% m/m) required for the tanning industry compared to the method reported [27]. Basic chromium sulfate product is nearly free from iron content when compared to the published methods. Thus, leaching with water is highly recommended for the production of basic chrome sulfate. PGMs were preconcentrated by liquid–liquid extraction using APDC as efficient complexing agent, recovered with HNO3 and ICP-OES and/or ICP-MS analyzed. Work is continuing to conduct the roasting process at different temperature, extraction of Ni from roast residue, quantitative extraction and recovery of PGMs from chromate liquor and production of the target compound basic chromium. Work is ongoing for: (1) studying the roasting process at different temperature; (2) Studying the effect of particle size of the chromite ore and the crushed roast; Assigning the extraction and recovery of Ni from chromite roast residue and PGEs from the chromate liquor and finally (3) Production of the target basic chromium sulfate with low cost approach.
References
Sanchez-Segado S, Makanyire T, Escudero-Castejon L, Hara Y, Jha A (2015) Reclamation of reactive metal oxides from complex minerals using alkali roasting and leaching – an improved approach to process engineering. Green Chem 17:2059–2080
Gervilla F, Proenza JA, Frei R, González-Jiménez JM, Garrido CJ, Melgarejo JC, Meibom A, Díaz-Martínez R, Lavaut W (2005) Distribution of platinum-group elements and Os isotopes in chromite ores from Mayarí-Baracoa Ophiolitic Belt (Eastern Cuba). Contrib Miner Pet 150:589–607
Uysal I, Sadiklar MB, Zaccarini F, Garuti G, Tarkian M, Meisel T (2010) The state of the art. In: 11th International platinum symposium, June 21–24, Ontario Geological Survey, Miscellaneous Release-Data 269
Uysal I, Tarkian M, Sadiklar MB, Zaccarini F, Meisel T, Garuti G, Heidrich S (2009) Petrology of Al- and Cr-rich ophiolitic chromitites from the Muğla, SW Turkey: implications from composition of chromite, solid inclusions of platinum-group mineral, silicate, and base-metal mineral, and Os-isotope geochemistry. Contrib Miner Petrol 158(5):659–674
Kozlu H, Rudashevsky VN (2010) “PGE in the 21st century: innovations in understanding their origin and applications to mineral exploration and beneficiation”, The state of the art. In: 11th International platinum symposium, June 21–24, Ontario Geological Survey, Miscellaneous Release-Data 269
Kapsiotis A, Grammatikopoulos T, Tsikouras B, Hatzipanagiotou K (2010) Platinum-group mineral characterization in concentrates from high-grade PGE Al-rich chromitites of Korydallos area in the Pindos ophiolite complex (NW Greece). Resour Geol 60(2):178–191
Parirenyatwa S, Escudero-Castejon L, Sanchez-Segado S, Yotamu Hara Y, Animesh Jha A (2016) Comparative study of alkali roasting and leaching of chromite ores and titaniferous minerals. Hydrometallurgy 165:213–226
Prichard HM, Neary CR, Fisher PC, O’Hara MJ (2008) PGE-rich podiform chromitites in the Al ‘Ays ophiolite complex, Saudi Arabia: an example of critical mantle melting to extract and concentrate PGE. Econ Geol 103:1507–1529
Harbi HM, Ali KA, Eldougdoug AA, Al-Jahdli NS (2016) Geochemistry and U-Pb zircon dating constraints of some plutonic rocks along Bir Tawilah shear zone, central Saudi Arabia: implication for magma peterogenesis and age of gold mineralization. Chem Erde 76:309–324
Basaran B, Ulas M, Bitlisli BO, Aslan A (2008) Distribution of Cr (III) and Cr (VI) in chrome tanned leather. Indian J Chem Technol 15:511–514
Saville J, Stamford CS (1981) Process for extraction of platinum group metals from chromite-bearing ore. United States Patent. http://www.patentstorm.US/patents/4295881
Wesseldijk QI, Reuter MA, Bradshow DJ, Harris PJ (1999) The flotation behavior of chromite with respect to the beneficiation of UG2 ore. Miner Eng 12(10):1177–1184
Cramer LA (2001) Minerals. Metals Mater Soc 53:14–18
Bryson MAW (2004) New technologies in the concentration of PGM values from UG-2 ores. J South Afr Inst Min Metall 104:311–313
Xiao Z, Laplante AR (2004) Characterizing and recovering the platinum group minerals—a review. Miner Eng 17:961–979
Barefoot RR, Van Loon JC (1999) Recent advances in the determination of the platinum group elements and gold. A Review. Talanta 49:1–14
Miezitis Y, Australia's Identified Mineral Resources (2017) Commonwealth of Australia (Geoscience Australia) 2017. ISSN: 1327-1466
Mpinga CN, Eksteen JJ, Aldrich C, Dyer L (2015) Direct leach approaches to platinum group metal (PGM) ores and concentrates. A Review. Miner Eng 78:93–113
Panda R, Jha MK, Pathak DD (2018) Commercial process for the extraction of platinum group metals (PGM). In: The Minerals, Metals & Materials Society, pp 119–130
Aghaei E, Alorro RD, Encila A, Yoo K (2017) Magnetic adsorbents for the recovery of precious metals from leach solutions and wastewater. Metals 7:529–560
Balcerzak M (2011) Methods for the determination of platinum group elements in environmental and biological materials: a review. Crit Rev Anal Chem 41(3):214–235
Balcerzak M (2002) Methods for the determination of platinum group elements in environmental and biological materials. Crit Rev Anal Chem 41(3):214–235
Merkle RKW, Loubser M, Gräser PPH (2004) Incongruent dissolution of chromite in lithium tetraborate flux. X Ray Spectrom 33:222–224
Chattopadhyay P, Mistry M (1996) Rapid chromite dissolution using manganese dioxide-lithium-sulphate-sulphuric acid mixture-estimation of alumina in chromite for quality control assessment. Microchem Acta 122:167–173
Narin, Soylak M, Dogan M (1997) Traffic pollution in Nigide-Turkiye: investigation of trace element contents. Fresenius Environ Bull 6:749–752
Antony MP, Tathavadkar VD, Calvert CC, Jha A (2001) The soda-ash roasting of chromite ore processing residue for the reclamation of chromium. Metall Mater Trans B 32B:987–995
Zhang B, Shi P, Jiang M (2016) Advances towards a Clean hydrometallurgical process for chromite. Minerals 6:1–7
Brooks RR, Hoashi M, Wilson SM, Zhang R-Q (1989) Extraction into methyl isobutyl ketone of metal complexes with ammonium pyrrolidine dithiocarbamate formed in strongly acidic media. Anal Chim Acta 217:165–170
Marczenko Z, Balcerzak (2000) Separation. Preconcentration and spectrophotometry in inorganic analysis, 1st edn. Elsevier
Totland MM, Jarvis I, Jarvis KE (1995) Microwave digestion and alkali fusion procedures for the determination of the platinum-group elements and gold in geological materials by ICP-MS. Chem Geol 124:21–36
Zientek ML (2012) Magmatic ore deposits in layered intrusions—descriptive model for reef-type PGE and contact-type Cu-Ni-PGE deposits U.S. geological survey open-file report 2012–1010, 48 p
Lomberg K (2014) Best practice sampling methods, assay techniques, and quality control with reference to the platinum group elements (PGEs). J South Afr Inst Min Metall 114:53–62
Zaccarini F, Bakker RJ, Garuti G, Aiglsperger T, Thalhammer OAA, Campos L, Proenza JA, Lewis JF (2010) Platinum group minerals in chromitite bodies of the Santa Elena Nappe, Costa Rica: mineralogical characterization by electron microprobe and Raman-spectroscopy. Bol Soc Geol Mex 62(1):161–171
Aslan N, Kaya H (2009) Investigation of the enrichment possibilities of Tekcrom mining company Tailings. Arab J Sci Eng 34(2):285–297
Jolly WL (1984) Modern inorganic chemistry. McGraw-Hill, New York
Chen X, Chen Y, Zhou T, Liu D, Hu H, Fan S (2015) Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries. Waste Manag 38:349–356
Dubiella-Jackowska A, Polkowska Z, Namiesnik J (2007) Platinum group elements. A challenge for environmental analysis. Polish J Environ Stud 16(3):329–345
Charlesworth P (1981) Separating the platinum group metals by liquid-liquid extraction. Platinum Met Rev 25(3):106–112
Bateman (2005) Extracting PGMs from chromite tailings at Kroondal – A world First. http://www.Bateman.co.za/globe50
Cawthorn RG (2002) 9th International platinum symposium, platinum metals rev., vol 46, no 4, pp 177–180
Funding
The authors would like to acknowledge the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah for technical support under Grant No. 391-145-1431.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
M. S. El-Shahawi: On sabbatical leave from the Department of Chemistry, Faculty of Science, Damiatta University, Damiatta, Egypt.
Rights and permissions
About this article
Cite this article
Eldougdoug, A.A., Harbi, H.M., Alwael, H. et al. Mineral processing: leaching process of chromium and recovery of platinum group elements from Northwestern Saudi Arabian Ophiolitic chromite. SN Appl. Sci. 1, 527 (2019). https://doi.org/10.1007/s42452-019-0471-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0471-y