Abstract
Mango fruits contain substantial vitamins and dietary fibre. Vitamins vary among and within fruits depending on cultivar type and ripening stage. Conventional techniques of vitamins analysis are based on High Pressure Liquid Chromatography, which are costly and laborious. This study evaluated the potential of Fourier transform infrared-diffuse reflectance spectroscopy (FTIR-DRIFTS) technique in predicting β-carotene, α-tocopherol and l-ascorbic acid in pulps of four mango cultivar types (‘Apple’, ‘Kent’, ‘Ngowe’, and ‘Tommy Atkins’). Combination of ran dom forest (RF) and first derivative spectra developed the predictive models. Factorial ANOVA examined the interaction effect of cultivar type, site (‘Thika’, ‘Embu’ and ‘Machakos), and fruit canopy position (sun exposed/within crown) on β-carotene, α-tocopherol and l-ascorbic acid contents. RF Models gave R2 = 0.97, RMSE = 2.27, RPD = 0.72 for β-carotene; R2 = 0.98, RMSE = 0.26, RPD = 0.30 for α-tocopherol and R2 = 0.96, RMSE = 0.51, RPD = 1.96 for l-ascorbic acid. Generally cultivar type affected vitamin C, F (3, 282) = 7.812, p < 0.05. Apple and Tommy Atkins had higher mean vitamins than Ngowe and Kent. In Machakos, within canopy fruits had higher β-carotene than sun-exposed fruits, F (5, 257) = 2.328, p = 0.043. However, interactions between fruit position, site and cultivar did not affect α-tocopherol and vitamin C. In Thika, Tommy Atkins at fully ripe stage had higher vitamin C than at intermediate maturity stage, F (2, 143) = 7.328, p = 0.01. These results show that FTIR-DRIFTS spectroscopy is a high-throughput method that can be used to predict mango fruit vitamins of in a large data set.
Similar content being viewed by others
1 Introduction
Mango is a climacteric fruit of commercial and nutritional importance in Kenya. Nutritionally, the fruit contains considerable quantities of carotenoids, α-tocopherol (vitamin E), l-ascorbic acid (vitamin C) and dietary fibre [1]. The presence of carotenoids in mango fruits has characterized skin and edible pulp colour, with exemptions to some red anthocyanins present in the fruit skin [2]. Some of the carotenoids in mango fruits includes α-carotene, β-carotene, γ-carotene and oxygenated carotenoids (xanthophyll’s) [3]. The carotenoids belong to a class of structurally related 40-carbon compounds made of eight repeating isoprene units [4]. Amongst these carotenoids, β-carotene is the predominant and acts as an important precursors of vitamin A [5]. The general recommended dietary allowance (RDA) for vitamin A among adult males and adult females are 900 μg/day and 700 μg/day respectively [6].
Similarly, vitamin E is reportedly present in mango pulps [7]. The vitamin E is composed of four tocopherols and four tocotrienols [8]. Tocopherols are derivatives of tocol and have a saturated side chain consisting of three isoprenoid units [8]. The antioxidant activities of the tocopherols and tocotrienols (grouped as chromanols) are associated with their ability to contribute phenolic hydrogens to lipids free-radicles [9]. Amongst these tocopherols, alpha-tocopherol (α-tocopherol) is used in calculation of recommended dietary allowance (RDA), in which for males and females of over 14 year, 15 μg of alpha-tocopherol per day is recommended [6].
In addition to the presence of carotenoids and α-tocopherol in mango fruit pulps, vitamin C, also known as l-ascorbic acid or acid (AA) is another predominant vitamin of mango fruit pulps. Ascorbic acid (AA) is labile and usually used as index to measure nutrient retention during processing effects on nutrient retention of fruits [10]. The presence of 2, 3-enediol moiety in AA structure makes the vitamins acidic and a strong reducing agent [11]. Consumption of 60 mg/day for adults meets the minimum dietary need [12]. However, the recommended dietary allowances (RDA) for adult men and women are 90 mg/day and 75 mg/day respectively [6] while, for children aged 9–12 years its 45 mg/day [6].
Previous studies by Mercadante and Rodriguez-Amaya [7] reported that vitamins contents of fruits are mostly affected by the type of cultivar, maturity stage, growing conditions (weather, growing season, intensity of sunlight, and soil state) as well as and manner of harvesting. Muoki et al. [5]. reported β-carotene contents for ripe mango fruits of Tommy Atkins and Ngowe cultivars in the range of 1.8–7.4 µg/g and 0.6–5.0 µg/g respectively. Ajila et al. [5]. reported vitamin E content of ‘Raspuri’ and ‘Badami’ mango cultivars of India in a range of 205–509 µg/g for ripe mangoes, but these results were for the peel and not the pulp. In Kenya, Okoth et al. [13] reported vitamin C content of ‘apple’ mango cultivars from Machakos location as ranging from 99.07 mg/100 g fresh weight (FW) to 109.35 mg/100 g (FW) for mature. At green maturity stage, mango fruits are regarded as having low β-carotene content compared to fully ripe fruits [5], However, in Kenya majority prefer mature-green fruits with small additions of hot pepper and salt [5].
The existing methods of vitamins; β-carotene, α-tocopherol and l-ascorbic acid analysis in mango fruits includes High Pressure Liquid Chromatography (HPLC) or other wet analytical techniques. These existing analytical methods are tedious, laborious and expensive for large scale analysis [14], besides, their application for rapid vitamins screening in the field is a challenge. Fourier transform infrared-diffuse reflectance spectroscopy (FTIR-DRIFTS) often coupled with chemometric tools is a method that we suggest could act as an alternative technique. The FTIR-DRIFTS technique is rapid allowing large samples to be analyzed within a short time [15]. Sample are scanned with infrared beam, giving rise to two types of reflected energy; specular and diffuse reflectance [16]. FTIR-DRIFTS spectroscopy has attached accessories that minimizes specular reflected energy while optimizing the collection of the diffuse reflected energy [16]. To offset interferences resulting from particles size differences, replaceable potassium bromide (KBr) or zinc selenide (ZnSe) detectors are used [17].
This study aimed at evaluating the potential of FTIR-DRIFTS calibration models for high-throughput predictions of β-carotene, α-tocopherol and l-ascorbic acid contents of four mango cultivars; ‘Apple’,’Ngowe’,’Kent’ and ‘Tommy Atkins’ from three sites ‘Thika’, ‘Embu’ and ‘Machakos’ in Kenya. In addition, we considered potential factors that influence mango fruit vitamins. These factors were; (i) the site from where we harvested the fruits, (ii) the fruit canopy position (sun-exposed or within-crown), (iii) cultivar type and (iv) maturity stage.
2 Materials and methods
2.1 Plant material, sample selection and reagents
The study experiment was carried out at three different farm orchards sites of Kenya Agriculture and Livestock Research Organization (KALRO) in Thika, Embu and Machakos counties in Kenya. Four mango cultivars which represents three different maturity types; early maturity between November to mid-January (Ngowe), mid maturity between late January to March (Tommy Atkins and Kent) and late maturity cultivar (Apple) [18, 19] were used. Five (5) fruit trees of each cultivar were randomly selected and ten (10) mango fruits identified and sampled per tree from two canopy positions; those that were exposed to sunlight (5) and those within the tree canopy (5).
In Thika, fruits were harvested at three different maturity stages. The sampled fruits were allowed to ripen at room temperature (20 °C), as determined by subjective observation of softness and peel color. A total of 480 fruits were sampled and freeze-dried, a representative sample selection algorithm, Kennard–Stone, was applied to further select samples for HPLC analysis [20]. The reagents used were standard β-carotene, α-tocopherol, l-ascorbic acid, Hyflosupercel (Celite), acetone, petroleum ether and Hexane (analytical grade); Acetonitrile, isopropanol and methanol (HPLC grade) all were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA), procured through Kobian, Kenya.
2.2 Extraction of vitamins and high pressure liquid chromatography (HPLC) analyses
2.2.1 β-carotene
The extraction procedure for β-carotene was carried out according to a protocol by Rodriguez-Amaya and Kimura [21]. Briefly, 1 g of freeze dried mango pulp was weighed, then transferred to a mortar and small amount (3 g) of Hyflosupercel (Celite) (Sigma-Aldrich Co., St. Louis, MO, USA), added. The sample was ground in this mixture with 50 mL of cold acetone then filtered with suction through a sintered glass funnel. Mortar, pestle, funnel, and residue were washed with small amounts of acetone, receiving the washings in the suction flask through the funnel until the residue or washings became devoid of color.
The extract was put in a 500 mL separatory funnel with Teflon stop-cock and 20 mL of petroleum ether (PE) added. Slowly distilled water (~ 100 mL) was added letting it flow along the walls of the funnel. The two phases were allowed to separate and aqueous phase discard. The PE phase was collected in a volumetric in 25 mL flask, making the solution pass through a small funnel containing anhydrous sodium sulfate (~ 15 g) to remove residual water.
The PE extract was centrifuged at 10,000 rpm for 10 min and then supernatant transferred to vial for sonication. The supernatant was dried under a stream of Nitrogen, and immediately before injection to HPLC (1200 system; Agilent Technologies, Hachioji-shi 1-9, Tokyo, Japan). It was reconstituted in 1 mL of PE. HPLC chromatographic conditions were set on monomeric C18 column: 3 µm, 4.6 × 150 mm with mobile phase of isopropanol: acetonitrile: methanol and an isocratic elution at 70:20:10, flow rate of 0.8 mL/min and injection volume of 5.0 μl. Calibration curves for β-carotene standard was established from four known concentrations as y = 15.596x − 21.168, R2 = 0.9997.
2.2.2 l-ascorbic acid
l-ascorbic acid analysis was done according to a modified method described by Lobo [22]. Approximately, 1 g (± 0.0001 g) of freeze-dried mango pulp was placed into 10 mL of 0.8% meta-phosphoric acid and ground with mortar and pestle. The extracts were filtered through Millipore filters (13 mm, 0.45 µm) and centrifuged at 10,000 rpm for 10 min.
Supernatants were held in ice and 10.0 μl of the supernatant injected to HPLC on monomeric C18 column with mobile phase of isopropanol: acetonitrile: methanol and an isocratic elution at 70:20:10, flow rate of 0.8 mL/min and injection volume of 10.0 μl. Calibration curves for l-Ascorbic acid was established from four known concentrations as y = 313531x − 12,164, R2 = 0.9642.
2.2.3 α-Tocopherol
A method described by Chun et al. [19]. was adopted and modified. Briefly, 0.5 g (± 0.0001 g) of freeze-dried mango pulp was weighed and placed in 50 mL beaker, 10 mL of hexane was added and the mixture placed on rotor votex for 15 min. The extracts were filtered through Millipore filters (13 mm, 0.45 µm) and centrifuged at 10,000 rpm for 15 min. 1 mL aliquot was put in Eppendorf tube for sonication (15 min).
The HPLC conditions were set on C18 column with mobile phase of isopropanol: acetonitrile: methanol and an isocratic elution at 50:30:20, flow rate of 0.8 mL/min and injection volume of 10.0 μl. Calibration curves for α-tocopherol was established from four known concentrations as y = 56359x − 50,916, R2 = 0.9687. The unit International unit (IU) not in milligrams (mg) is often used in the recommendations. One milligram of alpha-tocopherol equals to 1.5 International Units (IU).
2.2.4 Spectral acquisition and processing
Diffuse reflectance in 4000–400 cm−1 (2500–25,000 nm) region scanned approximately 2 mg of freeze-dried mango samples at 4 cm−1 resolution. Background spectrum correction was done using gold standard. The diffuse reflectance had an attached sampling accessory equipped with a sample holder cup (1.5 cm in diameter), on a Bruker Alpha spectrometer with a KBr detector (Bruker Optik GmbH, Ettlingen, Germany, available at World Agroforestry Center-Soil and Plant Spectral Laboratory). The acquired spectra were subjected to several spectral pre-processing algorithms including; Savitzky–Golay smoothing, multiplicative scatter correction (MSC), 1st and 2nd derivatives. However, 1st derivative pre-processing techniques which attempts to correct for baseline effects in spectra proved to be useful for this study.
2.2.5 Random forest (RF) ensemble
Random Forest (RF) [23] was used as a modelling tool for fruit vitamins predictions. The reference random forest method [23] is available in the R system for statistical computing version 3.1.0 [24] via the randomForest add-on package [19, 25]. RF works by fitting many classification trees to a data set, and then combines the predictions from all the trees [23]. The algorithm starts by selecting many bootstrap samples from the data set, and then fits a classification to each bootstrap sample, but at each node, only few randomly selected variables are made available for binary partitioning. The trees are then fully grown, and used to predict the out-of-bag observations. Predicted observations are calculated by majority vote of the out-of-bag predictions while the ties are split randomly.
Depending on the data set, number of predictors (mtry) and the number of trees to be built in the forest (ntree) can be varied [26]. Each tree is built from a bootstrap sample of the original data set which allows for robust error estimation with the remaining test set, the so-called Out-Of-Bag (OOB) sample. The excluded OOB samples are predicted from the bootstrap samples and by aggregating the OOB predictions from all trees, the Mean Square Error (MSEOOB) is calculated [25]. The mtry and ntree, were optimized based on root mean square error of validation (RMSEV). The ntree values were set at 1500, while mtry values were evaluated at all possible wavebands [27].
2.2.6 Model validation
A leave-one-out cross validation method was applied to assess the performance of the predictive models developed. Leave-one-out cross validation method works by dividing the data into k samples (k = total number of samples used for the analysis), and then the samples are removed one by one [28]. The model was calibrated k times using all k samples, except for the omitted one, and used to predict mango fruit vitamins on this excluded sample. One-to-one relationships between measured and predicted vitamin values were established and coefficients of determination (R2) as well as RMSEV values calculated.
A factorial ANOVA examined the interaction effect of cultivar type, site and fruit canopy position on β-carotene, α-tocopherol and l-ascorbic acid contents. Where a significant difference (p < 0.05) was detected, tukey’s means test was applied to evaluate the difference between the samples. In addition, independent sample t test compared the means from two fruit positions.
3 Results
3.1 High pressure liquid chromatography (HPLC)
β-carotene, α-tocopherol and l-ascorbic acid were detected at 5.29, 3.13 and 2.75 min respectively, for the chromatograms see supplementary information. β-carotene, α-tocopherol and l-ascorbic acid were identified by comparing the spectrum (λ max and fine structure) with those given in the literature and by co-elution with respective standards.
3.2 Spectral features
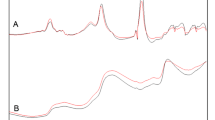
The FTIR spectra of mango fruit pulps are shown in Fig. 1. From the spectra, it looks difficult to make straight forward assignment of frequencies present in the spectra of the molecule. However, bands between 1250 and 740 cm−1 are characteristic of 7-cis configuration while those at 780 cm−1 are characteristic of 15-cis configuration. β-carotene vibrations results from its polyene backbone which includes C=C–C stretching, and C=C–C and C–C–C deformations. The β-carotene isomers exist as stretched (all-trans), terminal bent (7-cis) and central bent (15-cis) configurations. There is considerable coupling between C=C and C–C stretching with C–H in-plane bending and between C–H out-of-plane vibrations with C=C torsions (Fig. 1). Peaks at 1720 and 1680 are characteristic of C=C–C stretching. The α-tocopherol exhibits peaks at 2922 cm−1 (asymmetric stretch) and 2862 cm−1 (symmetric stretch) associated with vibrations of the CH2 and CH3 respectively (Fig. 1). The peaks at 2933 cm−1 and 2940 cm−1 are related to –CH stretch and the first –OH overtone of l-ascorbic acid.
3.3 Descriptive statistics
Applications of FTIR-DRIFTs spectroscopy to predict a range of vitamins from a range of mango cultivars are outlined in Table 1. β-carotene contents showed significant variations in the calibration dataset; CV = 30.66% with Min = 3.44 g/100 g and Max = 14.83 g/100 g freeze dry weight (FDW). Different spectral pretreatment methods were tested, although first derivative spectral pre-treatments gave optimal models for β-carotene, α-tocopherol and l-ascorbic acid.
3.4 Random forests ensemble models
Predictions from random forest produced good results for all the vitamins (Table 2). The R2 and RMSE values for cross-validation of β-carotene were R2 = 0.97, RMSE = 2.27, RPD = 0.72; α-tocopherol, R2 = 0.98, RMSE = 0.26, RPD = 0.30 and l-ascorbic acid, R2 = 0.96, RMSE = 0.51, RPD = 1.96 (Table 2). The measured versus predicted vitamin values from the cross-validation set for these models are shown in Fig. 2. The RPD values of β-carotene suggested quality models. Regarding the β-carotene, α-tocopherol and ascorbic acid of mango fruit pulp, the RF model estimate resulted in good estimation, as is observed by the bias values (Fig. 2).
Measured versus predicted vitamin values from the mid-infrared diffuse reflectance spectra as determined by random forests ensemble tree regression. Coefficient of determination (R2), root mean squared error (RMSE) and residual prediction deviation (RPD) are given in Table 2
3.5 Random forest (RF) predictive models
From the analysis of entire predicted datasets, beta-carotene mean content was 8.02 mg/100 FDW with a minimum of 5.18 mg/100 FDW and a maximum of 8.19 mg/100 FDW, vitamin C was 3.23 mg/100 FDW with a range of 2.69–3.88 mg/100 FDW, while α-tocopherol ranged from 2.89 mg/100 FDW to 3.41 mg/100 FDW with a mean of 3.13 mg/100 FDW (Fig. 3). Ascorbic acid (vitamin C) content varied significantly within mango cultivars. The maximum value for β-carotene was detected in the pulp of mango cv. Apples (8.19 ± 1.06 mg/100 g FDW), followed by Kent cv. (7.95 ± 1.14 mg/100 g FDW) while, Ngowe cv. had the lowest β-carotene content (vitamin A) percentage (7.86 ± 1.00 mg/100 g FDW).
Comparisons of mean vitamins for; Apple, Tommy Atkins, Ngowe and Kent mango cultivars across Thika, Machakos and Embu sites. *Three factorial ANOVA followed by LSD and Turkey test. Values are lettered in descending order of size where significant difference was seen (p < 0.05). Amounts are g/100 g FDW, N = number of trees per cultivar in each site
When the effect of site, cultivar type and fruit position on the vitamin contents of mangoes was tested by performing factorial ANOVA, results showed that site had no significant effect on β-carotene, α-tocopherol and vitamin C content, p > 0.5. The cultivar type on the other hand only had an effect on vitamin C content F (3, 282) = 7.812, p < 0. 05, η2 = 0.077 with Apple and Tommy Atkins cultivars having significantly higher mean vitamins than Ngowe and Kent cultivars. Interactions between cultivar type and site affected l-Ascorbic acid content; F (5, 257) = 4.100, p = 0.00, η2 = 0.074 and Tocopherol; F (5, 257) = 6.484, p = 0.00, η2 = 1.112. Tocopherol was also affected by the interaction between cultivar type and fruit position; F (3, 257) = 3.274, p = 0.022, η2 = 1.037. In general the effect size of site, cultivar type, fruit position and their interactions on the vitamin content was small as indicated by small partial eta squared values (η2 < 0.5).
When each cultivar type was considered separately (Fig. 3), apple cultivars from Machakos site (M = 3.32) and Embu site (M = 3.27) had significantly higher l-ascorbic acid content than similar apple cultivars from Thika site (M = 3.15). Kent cultivars from Machakos and Thika sites had significantly high α-tocopherol content compared to Kent cultivars from Embu site. In contrary, α-tocopherol content of Tommy Atkins cultivars from Thika site (M = 3.20) and Embu site (M = 3.17) were significantly higher than those of Tommy Atkins cultivars from Machakos site (M = 3.10), (Fig. 3). However, a part from Apple cultivars, no significant differences in vitamin C content of Kent and Tommy Atkins cultivars from different sites (p > 0.05) was observed. But in a general trend, vitamin C of these four cultivars, were mostly affected by site.
3.6 Fruit canopy position and the vitamins contents
β-carotene content in Tommy Atkins cultivars from Machakos site were affected by the fruit position; mango fruits found within the tree crown had significantly higher β-carotene content than the sun-exposed fruits, F (5, 257) = 2.328, p = 0.043, η2 = 0.043. Fruits of Kent mango cultivars from Thika site that were sun-exposed had significantly higher α-tocopherol content compared to those fruits within the tree canopy (Fig. 4). However, no significant difference in mean α-tocopherol, F (5, 257) = 0.878, p = 0.496, η2 = 0.017 and l-ascorbic acid, F (5, 257) = 2.089, p = 0.067, η2 = 0.039 content resulting from interaction between fruit position, site and cultivar type was observed.
In a general trend, when all the cultivars were pooled together regardless of the site, β-carotene content was significantly higher amongst within the tree crown fruit pulps (8.15 ± 1.03) than sun-exposed (7.75 ± 1.11) fruit pulps as determined by T test; t (482) = − 4.107, p = 0.00 (Table 3). A similar trend was observed for l-ascorbic acid (t (481) = -2.404, p = 0.017).
When comparison of mean individual vitamin content within different fruit cultivars were computed, β-carotene content of Tommy Atkins cultivar was significantly higher among fruits found within the tree canopy compared to those that were sun exposed (t (144) = − 2.56, p = 0.01). Additionally, α-tocopherol content of sun-exposed Tommy Atkins were higher than for fruits positioned within the tree canopy as determined by T test, t (144) = 1.967, p = 0.05. For Ngowe cultivar; mean β-carotene (t (87) = -3.495, p = 0.00) and l-ascorbic acid (t (86) = -3.497, p = 0.00) content was significantly higher in the within-the- canopy fruits than sun-exposed fruits (Fig. 4). However, for Apple cultivars, no significant difference in β-carotene, α-tocopherol and l-ascorbic acid contents were observed for both the sun-exposed and within the tree crown fruits. While Kent cultivar showed a significant difference only for α-tocopherol contents which were higher for the within the canopy fruits than those of sun-exposed fruits (t (96) = − 2.694, p = 0.01) (Fig. 4).
3.7 Fruit maturity stage and cultivar type on vitamins contents
The effect of maturity stage and the interactions between the maturity stage and fruit position on the vitamins content was tested in Apple, Kent, Ngowe, and Tommy Atkins cultivars from Thika site. From the results, fully ripe Tommy Atkins mangoes had significantly high l-ascorbic acid contents compared to similar cultivar fruits at intermediate maturity stage, (F (2, 143) = 7.328, p = 0.01) (Fig. 5). However, no significant difference in l-ascorbic acid content of Tommy Atkins fruits at green maturity and fully ripe/intermediate stage were observed (Fig. 5).
Ngowe cultivars showed no significant difference in the mean β-carotene, α-tocopherol and l-ascorbic acid concentration at different fruit maturity stages. While for Apple cultivars; l-ascorbic acid was significantly higher at intermediate maturity stage fruits than at fully ripe stage (F (2, 148) = 6.288, p = 0.02) (Fig. 5). Kent cultivars showed higher β-carotene and l-ascorbic acid contents at green maturity stage than at the intermediate or fully ripe stage. For the same Kent cultivars, α-tocopherol contents were high at intermediate maturity stage compared to fully ripe or green mature stage (F (2, 95) = 9.081, p = 0.00) (Fig. 5).
The interaction between the stage of maturity and the fruit position only affected the content of β-carotene; F (2, 257) = 0.233, p = 0.043, η2 = 0.002. As indicated by the small partial eta squared value (η2 = 0.002), the effect size was small. The general trend of β-carotene, α-tocopherol, and l-ascorbic acid of mango fruits of all cultivars increased from green maturity stage to fully ripe stage is illustrated in Fig. 6.
4 Discussions
4.1 Spectral characteristics of vitamins and RandomForest Predictive Models
Previous studies have indicated that robust models using FTIR-DRIFTs spectra can be generated for β-carotene, α-tocopherol and l-ascorbic acid. The β-carotene spectrum exhibited peaks at 2922 cm−1 and 2862 cm−1 for asymmetric and symmetric stretching vibrations of the CH2 and CH3 respectively (Fig. 1). Upon examination of FTIR-DRIFTs spectra for α-tocopherols, unique bands around 880–785 cm−1 region were observed (Fig. 1). These bands could possibly arise from vibrations of the benzopyrane ring structure [29]. However, absorptions around 1500–1000 cm−1 region are characteristics of tocopherols [29]. The major peaks in these spectra arise from C=O stretching vibrations (1746 cm−1), CH3 and CH2 scissoring vibrations (1377 and 1467 cm−1), C–O stretching vibrations (1118, 1163 and 1238 cm−1) and CH2 rocking modes (723 cm−1) according to previous work on tocopherols [29].
As previously reported in Table 2, all of the calibration models had high R2 range; R2 = 0.96-0.98. These values were within the ranges reported by other authors; R2 = 0.95-0.98 for β-carotene [30], R2 = 0.95 for α-tocopherol [31] and R2 = 0.98 for l-ascorbic acid [32]. RPD values were also satisfactory. Optimal mtry values for RF regression models indicated that adjusting mtry values did not further improve RMSE.
4.2 Changes in fruit vitamin contents with tree canopy position
The average β-carotene, α-tocopherol and l-ascorbic acid contents in the pulp of the four mango cultivars harvested from two fruit position; sun-exposed and within the tree canopy fruits are listed in Table 3. Generally, fruits that are sun-exposed receive higher light intensity with relatively more UV and red light than in other positions [33, 34]. Similarly, in this study, better light conditions experienced in sun-exposed fruits resulted in significantly high l-ascorbic acid contents of mango fruits (Fig. 4).
On contrary, β-carotene content of sun-exposed fruits was slightly lower than those fruits found within the tree canopy (Table 3). These findings for β-carotene are in agreement with those previously reported by Verreynne et al. [35], who reported no qualitative change in the carotenoid content of mango fruits which were either sun-exposed or within the tree crown. However, the results disagree with those reported by other authors [35, 36] who found that fruits exposed to sunlight were redder in pulp than those within the canopy.
These disagreements might be explained by the fact that majority of the sampled mango trees had low fruit load in the trees; this fact allowed fruits that were sun-exposed or within the crown to have access to sunlight. Better light conditions are known to induce anthocyanin synthesis consequential increasing the levels of quercetin glycosides and cyanidin glycosides in the skin of sun-exposed fruits which in turn gives better fruit colouration [33, 36]. Previous studies by Tustin et al. [37]. found light exposure as a factor affecting fruit vitamins as well as dry matter accumulated by fruits hence the trees’ productivity in terms of the fruit and the vegetative growth [37]. However, among the vitamins, α-tocopherol content did not show significant acclimation to light exposure when all cultivars were polled together (Table 3).
Additionally, the differences in β-carotene, α-tocopherol and l-ascorbic could be due to variations in climatic conditions and cultivar differences.
4.3 Changes in fruit vitamins contents with ripening stage
As reported in Fig. 5, there was a general trend of increase in β-carotene and α-tocopherol contents as ripening stage progressed from mature green to fully ripe stage. These results are similar to those reported by Ornelas-Paz et al. [38]. and Robles-Sánchez et al. [39] in ‘Manila’ and ‘Ataulfo’ mangos respectively and even for sugars [34]. The increase in β-carotene with ripening stage has been associated with the increase in respiration initiated by the action of ethylene produced (Mercadante and Rodriguez-amaya 1998).
Previous studies by Mercadante and Rodriguez-amaya [7] on carotenoids, reported that amongst other factors; mango fruits are affected by the stage of maturity, harvesting conditions and fruit processing techniques. It is well defined that β-carotene as a terpenoid, depends on harvest stage because the biosynthesis of terpenes decreases with fruit maturity [40]. However, mango fruits have been reported to accumulate tocopherols and carotenoids during ripening [41, 42]. The synergistic effect between vitamin C and vitamin E is well known [43], as a result, it is possible that the presence of vitamin C in mangos contributed to retardation of vitamin E oxidation, but was not sufficient to avoid losses during pulp storage [43]. Being lipophilic antioxidant, α-tocopherol has been reported to interact with carotenoid radical cations [44].
These interactions of α-tocopherol and carotenoid radical cations has resulted to the regeneration of parent carotenoid, thus preserving carotenoid colour and antioxidant activity [44]. Because of these interactions, α-tocopherol behaviour with regards to ripening stage is suggested to follow similar path to those of carotenoids. l-ascorbic acid decreased at the intermediate stage then increased at fully ripe stage, showing a significant change in the content. The mean l-ascorbic content decreased from 3.19 mg/100 g to 3.08 mg/100 g from mature-green stage to intermediate stage (Fig. 5).
These results on l-ascorbic acid are consistent with those published elsewhere [45, 46], who accordingly associated the decrease to candidate substrates formed during respiration. However, increase in l-ascorbic acid at some stages could probably be associated with the contribution of dehydroascorbic acid included in calculations of total vitamin C content in this work.
Both dehydroascorbic acid and l-ascorbic acid either in oxidized and reduced forms are nutritionally valuable [47]. Whereas vitamin C is abundantly available in many fruits and contributes essentially to antioxidant activity; the total antioxidant effect in a fruit system is due to the synergism between the various bioactive compounds in the fruit namely tocopherols, carotenoids, polyphenols, and micro elements [48].
5 Conclusions
In this study, RF ensemble classification approach using FTIR-DRIFT spectra was used to quantitatively predict β-carotene, α-tocopherol and l-ascorbic contents of four mango cultivars coming from four sites. This analytical approach, when combined with chemometric tools and calibration data sets carefully selected, could generate accurate prediction data and result in successful implementation of FTIR-DRIFTs in monitoring the intrinsic quality nutritional attributes of mango fruits and other neglected fruits. In addition, same spectra can be used on several parameters predictions. The differences observed between mango fruits from two canopies position; sun-exposed and within crown, could also be as a result of physiological age of fruits at harvest time, cultivar type and site. To minimize these effects; pruning of mango trees regardless of cultivar type must be encouraged. Moreover, the progressive increase in β-carotene and α-tocopherol content during ripening stages (mature green stage to fully ripe stage) should help in offering guidelines during harvest/storage, thus help the farmers maintain mango fruit quality over prolonged storage life.
References
Pal RK (1998) Ripening and rheological properties of mango as influenced by ethene and calcium carbide. J Food Sci Technol 35:358–360
Beutner S, Bloedorn B, Frixel S et al (2001) Quantitative assessment of antioxidant properties of natural colorants and phytochemicals: carotenoids, flavonoids, phenols and indigoids. The role of β-carotene in antioxidant functions. J Sci Food Agric 81:559–568. https://doi.org/10.1002/jsfa.849
Ben-Amotz A, Fishler R (1998) Analysis of carotenoids with emphasis on 9-cis beta-carotene in vegetables and fruits commonly consumed in Israel. Food Chem 62:515–520. https://doi.org/10.1016/S0308-8146(97)00196-9
Boon CS, McClements DJ, Weiss J, Decker EA (2010) Factors influencing the chemical stability of carotenoids in foods. Crit Rev Food Sci Nutr 50:515–532. https://doi.org/10.1080/10408390802565889
Muoki PN, Makokha AO, Onyango CA, Ojijo NK (2009) Potential contribution of mangoes to reduction of vitamin a deficiency in kenya. Ecol Food Nutr 48:482–498. https://doi.org/10.1080/03670240903308604
IOM (2000) Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. National Academy Press, Washington, DC
Mercadante AAZ, Rodriguez-Amaya DDB (1998) Effects of ripening, cultivar differences, and processing on the carotenoid composition of mango. J Agric Food Chem 46:128–130. https://doi.org/10.1021/jf9702860
DeMan JM (1999) Carbohydrates. Principles of food chemistry, 3rd ed. Aspen Publishers, Inc, Gaithersburg, pp 163–183
Kamal-Eldin A, Appelqvist L-Å (1996) The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 31:671–701. https://doi.org/10.1007/BF02522884
Vanderslice JT, Higgs DJ, Hayes JM, Block G (1990) Ascorbic acid and dehydroascorbic acid content of foods-as-eaten. J Food Compos Anal 3:105–118
Liao M-L, Seib PA (1988) Chemistry of l-ascorbic acid related to foods. Food Chem 30:289–312
Taylor CA, Hampl JS, Johnston CS (2000) Low intakes of vegetables and fruits, especially citrus fruits, lead to inadequate vitamin C intakes among adults. Eur J Clin Nutr 54:573–578. https://doi.org/10.1038/sj.ejcn.1601059
Okoth EM, Sila DN, Onyango CA et al (2013) Evaluation of chemical and nutritional quality attributes of selected mango varieties at three stages of ripeness, grown in lower Eastern province of Kenya—part 2. J Anim Plant Sci 17:2619–2630
Liu Y, Ying Y, Yu H, Fu X (2006) Comparison of the HPLC method and FT-NIR analysis for quantification of glucose, fructose, and sucrose in intact apple fruits. J Agric Food Chem 54:2810–2815
Duarte IF, Antonio B, Delgadillo I et al (2002) Application of FTIR Spectroscopy for the quantification of sugars in mango juice as a function of ripening. J Agric Food Chem 50:3104–3111
Bruno T (1999) Sampling accesories for infrared spectrometry. Appl Spectrosc Rev 34:91–120
Haberhauler G, Gerzabek MH (1999) DRIFT and Transmission FT-IR spectroscopy of forest soils: an approach to determine decomposition processes of forest litter. Vib Spectrosc 19:413–417
Njuguna JK, Wepukhulu SB, Wanjala S (2009) Mango cultivar evaluation programme in Kenya. Acta Hortic 820:133–135
Griesbach J (2003) Mango growing in Kenya. World Agroforestry Center, Nairobi
Kennard RWW, Stone LA (1969) Computer aided design of experiments. Technometrics 11:137–148
Rodriguez-Amaya DB, Kimura M (2004) Harvestplus handbook for carotenoid analysis. International Food Policy Research Institute (IFPRI) and International Center for Tropical Agriculture (CIAT, Washington, DC and Cali
Hernández Y, Lobo MG, González M (2006) Determination of vitamin C in tropical fruits: a comparative evaluation of methods. Food Chem 96:654–664. https://doi.org/10.1016/j.foodchem.2005.04.012
Breiman L (2001) Random forests. Mach Learn 45:5–32. https://doi.org/10.1023/A:1010933404324
R Development Core Team (2014) R: a language and environment for statistical computing. 2673
Liaw A, Wiener M (2002) Classification and regression by random forest. R News 2:18–22. https://doi.org/10.1177/154405910408300516
Wiesmeier M, Barthold F, Blank B, Kögel-knabner I (2011) Digital mapping of soil organic matter stocks using Random Forest modeling in a semi-arid steppe ecosystem. Plant Soil 340:7–24. https://doi.org/10.1007/s11104-010-0425-z
Díaz-Uriarte R, De Andrés SA (2006) Gene selection and classification of microarray data using random forest. BMC Bioinform 7:3
Abdel-Rahman EM, Ahmed FB, Ismail R (2013) Random forest regression and spectral band selection for estimating sugarcane leaf nitrogen concentration using EO-1 Hyperion hyperspectral data. Int J Remote Sens 34:712–728. https://doi.org/10.1080/01431161.2012.713142
Guillen M, Cabo N (1997) Infrared spectroscopy in the study of edible oils and fats. J Sci Food Agric 75:1–11
Chen X, Wu J, Zhou S et al (2009) Application of near-infrared reflectance spectroscopy to evaluate the lutein and β-carotene in Chinese kale. J Food Compos Anal 22:148–153. https://doi.org/10.1016/j.jfca.2008.10.007
Ahmed MK, Daun JK, Przybylski R (2005) FT-IR based methodology for quantitation of total tocopherols, tocotrienols and plastochromanol-8 in vegetable oils. J Food Compos Anal 18:359–364. https://doi.org/10.1016/j.jfca.2003.12.008
Suhandy D, Yulia M, Ogawa Y, Kondo N (2012) l-Ascorbic acid prediction in aqueous solution based on FTIR-ATR terahertz spectroscopy *. Eng Agric Environ Food 5:152–158
Jakopic J, Stampar F, Veberic R (2009) The influence of exposure to light on the phenolic content of “Fuji” apple. Sci Hortic (Amsterdam) 123:234–239. https://doi.org/10.1016/j.scienta.2009.09.004
Olale K, Walyambillah W, Mohammed SA, et al (2017) Application of DRIFT-FTIR spectroscopy for quantitative prediction of simple sugars in two local and two Floridian mango (Mangifera indica L.) cultivars in Kenya. J Anal Sci Technol 8:21. https://doi.org/10.1186/s40543-017-0130-0
Verreynne JS, Rabe E, Theron KI (2004) Effect of bearing position on fruit quality of mandarin types. South African J Plant Soil 21:1–7. https://doi.org/10.1080/02571862.2004.10635014
Bible B, Singha S (1993) Canopy position influences CIELAB coordinates of peach color. HortScience 28:992–993
Tustin D, Hirst P, Warrington I (1998) Influence of orientation and position of fruiting laterals on canopy light penetration, yield, and fruit quality of ‘Granny Smith’ apple. J Am Soc Hort Sci 113:693–699
Ornelas-Paz J, Yahia E, Gardea A (2008) Changes in external and internal color during postharvest ripening of Manila and Ataulfo mango fruit and relationship with carotenoid content determined by liquid chromatography- APcI + -time-of-flight mass spectrometry. Postharvest Biol Technol 50:145–152
Robles-Sánchez RMR, Rojas-Graü MA, Odriozola-Serrano I et al (2009) Effect of minimal processing on bioactive compounds and antioxidant activity of fresh-cut Kent mango (Mangifera indica L.). Postharvest Biol Technol 51:384–390. https://doi.org/10.1016/j.postharvbio.2008.09.003
Joas J, Vulcain E, Léchaudel M (2013) Effect of fruit position in the canopy on physiological age and physicochemical composition of mango ‘Cogshall’. In: IXth International. Mango symposium. Acta Horticulturae. pp 123–128
Ajila CM, Bhat SG, Rao UJSP, Prasada R (2007) Valuable components of raw and ripe peels from two Indian mango varieties. Food Chem 102:1006–1011. https://doi.org/10.1016/j.foodchem.2006.06.036
Burns J, Fraser P, Bramley P (2003) Identification and quantification of carotenoids, tocoferols and chlorophyll in commonly consumed fruits and vegetables. Phytochemistry 62:939–947
Niki E, Noguchi N (2004) Dynamics of antioxidant action of vitamin E. Acc Chem Res 37:45–51
Mortensen A, Skibsted LH, Truscott TG (2001) Minireview: the interaction of dietary carotenoids with radical species. Arch Biochem Biophys 385:13–19
Tovar B, García H, Mata M (2001) Physiology of pre-cut mango. II. Evolution of organic acids. Food Res Int 34:705–714
Tovar B, Garca H, Mata M (2000) Physiology of mango.II. Evolution of organic acids. Physiol Mango Evolu Org Acids 21:39–49
Rivera-López J, Vázquez-Ortiz F, Ayala-Zavala JF et al (2005) Cutting shape and storage temperature affect over- all quality of fresh-cut papaya cv. ‘Maradol’. J Food Sci 70:482–489
Masibo M, He Q (2009) Mango bioactive compounds and related nutraceutical properties—a review. Food Rev Int 4:346–370. https://doi.org/10.1080/87559120903153524
Acknowledgements
The authors wish to thank Prof. Baldwin Torto of the International Centre of Insect Physiology and Ecology (ICIPE) for providing HPLC facilities. We also acknowledge contributions from Dr. Katja Kehlenbeck, Mr. Njuguna of KALRO-Kandara and Mr. John Wanangwe. To them all, we are very grateful.
Funding
This study was supported by World Agroforestry Centre under Agriculture for Nutrition and Health (A4NH) and The National Commission for Science, Technology and Innovation (NACOSTI/RCD/ST&I 5TH CALL, PhD/014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Olale, K., Walyambillah, W., Mohammed, S.A. et al. FTIR-DRIFTS-based prediction of β-carotene, α-tocopherol and l-ascorbic acid in mango (Mangifera indica L.) fruit pulp. SN Appl. Sci. 1, 279 (2019). https://doi.org/10.1007/s42452-019-0297-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0297-7