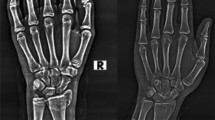
Abstract
Wrist fractures are currently examined using radiograms, resulting in undesirable radiation exposure in children. Ultrasound is fast, safe, and highly sensitive to fractures, making it ideally suited for wrist examination in emergency departments (ED). However, ultrasound images are difficult to interpret, resulting in high variability in assessment depending on the reader’s expertise. We developed a new machine learning (ML) technique to detect fractures from 3D ultrasound (3DUS). We generate bone probability maps using local phase (LP) information in each ultrasound frame, combine these into a feature sequence, and analyze the same to predict the probability of fracture using three variants of recurrent neural networks (RNN): vanilla RNN, long short-term memory (LSTM), and gated recurrent unit (GRU) models. This approach was validated on 30 3DUS volumes, each of which was assessed by a radiologist for the presence of a fracture. RNN, LSTM and GRU gave 83%, 90%, and 87% accuracy when compared to clinical assessment by expert musculoskeletal radiologist, with GRU giving the most balanced sensitivity and specificity. The automatic assessment technique is reliable in detecting wrist fractures from 3D ultrasound and can be used as a valuable ED triage tool for fracture detection.





Similar content being viewed by others
Data Availability
The ultrasound data used in this study is not available for public access as institutional policy.
Code Availability
The source code is not available for public access in line with our IP policy.
References
Baka N, Leenstra S, van Walsum T. Ultrasound aided vertebral level localization for lumbar surgery. IEEE Trans Med Imaging. 2017;36(10):2138–47.
Cao K, Mills DM, Thiele RG, Patwardhan KA. Toward quantitative assessment of rheumatoid arthritis using volumetric ultrasound. IEEE Trans Biomed Eng. 2016;63(2):449–58.
Cho K, van Merrienboer B, Bahdanau D, Bengio Y. On the properties of neural machine translation: encoder-decoder approaches. arXiv preprint arXiv:1409.1259. 2014. http://arxiv.org/abs/1409.1259. Accessed 21 Nov 2023.
Foroughi P, Boctor E, Swartz MJ, Taylor RH, Fichtinger G. P6D-2 ultrasound bone segmentation using dynamic programming. In: 2007 IEEE Ultrasonics Symposium Proceedings, 28 Oct 2007. IEEE; 2007. pp. 2523–6.
Hacihaliloglu I. Enhancement of bone shadow region using local phase-based ultrasound transmission maps. Int J Comput Assist Radiol Surg. 2017;12(6):951–60.
Hacihaliloglu I, Rasoulian A, Rohling RN, Abolmaesumi P. Local phase tensor features for 3-D ultrasound to statistical shape+pose spine model registration. IEEE Trans Med Imaging. 2014;33(11):2167–79.
Hareendranathan AR, Mabee M, Punithakumar K, Noga M, Jaremko JL. A technique for semiautomatic segmentation of echogenic structures in 3D ultrasound, applied to infant hip dysplasia. Int J Comput Assist Radiol Surg. 2016;11(1):31–42.
Hareendranathan AR, Mabee M, Punithakumar K, Noga M, Jaremko JL. Toward automated classification of acetabular shape in ultrasound for diagnosis of DDH: contour alpha angle and the rounding index. Comput Methods Programs Biomed. 2016;129(June):89–98.
Hareendranathan AR, Tripathi A, Panicker MR, Zhou Y, Knight J, Jaremko JL. Domain-aware contrastive learning for ultrasound hip image analysis. Comput Biol Med. 2022;149(October): 106004.
Hareendranathan AR, Zonoobi D, Mabee M, Diederichs C, Punithakumar K, Noga M, Jaremko JL. Semiautomatic classification of acetabular shape from three-dimensional ultrasound for diagnosis of infant hip dysplasia using geometric features. Int J Comput Assist Radiol Surg. 2017;12(3):439–47.
Hochreiter S, Schmidhuber J. Long short-term memory. Neural Comput. 1997. https://doi.org/10.1162/neco.1997.9.8.1735.
Jia R, Mellon S, Monk P, Murray D, Alison Noble J. A computer-aided tracking and motion analysis with ultrasound (CAT & MAUS) system for the description of hip joint kinematics. Int J Comput Assist Radiol Surg. 2016;11(11):1965–77.
Kim B, Kim KC, Park Y, Kwon J-Y, Jang J, Seo JK. Machine-learning-based automatic identification of fetal abdominal circumference from ultrasound images. Physiol Meas. 2018;39(10): 105007.
Pandey PU, Quader N, Guy P, Garbi R, Hodgson AJ. Ultrasound bone segmentation: a scoping review of techniques and validation practices. Ultrasound Med Biol. 2020;46(4):921–35.
Ronneberger O, Fischer P, Brox T. U-Net: convolutional networks for biomedical image segmentation. In: Medical Image Computing and Computer-Assisted Intervention–MICCAI 2015: 18th International Conference, Munich, Germany, 5–9 October 2015. Proceedings, Part III 18 2015. Springer International Publishing; 2015. pp. 234–41.
Salehi M, Prevost R, Moctezuma JL, Navab N, Wein W. Precise ultrasound bone registration with learning-based segmentation and speed of sound calibration. In: Medical Image Computing and Computer-Assisted Intervention−MICCAI 2017: 20th International Conference, Quebec City, QC, Canada, 11–13 September 2017. Proceedings, Part II 20 2017. Springer International Publishing; 2017. pp. 682–90.
Villa M, Dardenne G, Nasan M, Letissier H, Hamitouche C, Stindel E. FCN-based approach for the automatic segmentation of bone surfaces in ultrasound images. Int J Comput Assist Radiol Surg. 2018;13(11):1707–16.
Zhou Y, Rakkunedeth A, Keen C, Knight J, Jaremko JL. Wrist ultrasound segmentation by deep learning. In: International Conference on Artificial Intelligence in Medicine, 14 June 2022. Cham: Springer International Publishing; 2022. pp. 230–7.
Acknowledgements
Dr. J.J is supported by the AHS Chair in Diagnostic Imaging and Canada CIFAR AI Chair, and his academic time is made available by the Medical Imaging Consultants (MIC), Edmonton, Canada. We acknowledge the support of the TD Ready Health and Alberta Machine Intelligence Institute (AMII) for funding this project, Alberta Emergency Strategic Clinical Network and Alberta Innovates for clinical scanning, and Compute Canada in providing us with computational resources including high-power graphical processing units (GPU) that were used for training and testing our deep learning models.
Funding
This research was funded by the TD Ready Health Grant and Alberta Machine Intelligence Institute (AMII).
Author information
Authors and Affiliations
Contributions
ARH: conceptualization, literature review, methodology, software development, data analysis, writing — original draft preparation. AT: software development, visualization, writing — review and editing. MRP: conceptualization, methodology, software development, writing — review and editing. JZ: data collection, conceptualization, writing — review and editing. NB: data collection, conceptualization, writing — review and editing JJ: project supervision, conceptualization, funding acquisition, writing — review and editing.
Corresponding author
Ethics declarations
Ethics Approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of University of Alberta Hospital.
Consent to Participate
Informed consent was obtained from all subjects involved in the study.
Consent for Publication
All authors have agreed to publish this work in its current form.
Competing Interests
The authors of this manuscript declare relationships with the following companies: J. J was a co-founder of MEDO.ai Inc. — a company that develops AI-based solutions in medical ultrasound, which has since been acquired. Other authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Imaging
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hareendranathan, A.R., Tripathi, A., Panicker, M.R. et al. Deep Learning Approach for Automatic Wrist Fracture Detection Using Ultrasound Bone Probability Maps. SN Compr. Clin. Med. 5, 276 (2023). https://doi.org/10.1007/s42399-023-01608-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-023-01608-8