Abstract
Currently, pandemic coronavirus disease 2019 (COVID-19) is the biggest threat to all human beings globally. Till June 8, 2020, it has infected 6,931,000 people and caused 400,857 deaths worldwide. The first case was identified in a patient with influenza-like symptoms along with severe acute respiratory syndrome in Wuhan, China, in December 2019 and now it has spread in more than 200 countries. Since there is no approved cure for this disease until now, there is a lot of mass fear, apprehensions, and questions globally regarding (i) genetic origin and history of the novel coronavirus, (ii) what are the first-line therapies for those who contract this disease, and (iii) what could be the potential vaccine targets. In this short review, we have tried to address these queries in the simplest manner and compiled the history of previous coronaviruses, recent developments in the COVID-19 research, potential future therapeutics, and possible targets to cure the disease.
Similar content being viewed by others
Introduction
The novel coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a public health emergency, posing a serious threat to people’s health as well as the medical and health system worldwide. On February 11, 2020, World Health Organization named disease caused by a novel coronavirus (2019-nCoV) as coronavirus disease 2019 (COVID-19) [1, 2]. Initially, this was identified in three patients, who were hospitalized in Wuhan, China, from December 2019 to January 2020. The ailment which had likely been caused by this novel coronavirus (nCoV) was named “Novel Coronavirus-Infected Pneumonia” (NCIP). Genome sequence analysis revealed that nCoV falls into the genus Betacoronavirus and subgenus Sarbecovirus which also includes coronaviruses (SARS-CoV, bat-SARS-like CoV, and others) discovered in humans, bats, and other wild animals [3,4,5,6,7].
Coronaviruses contain RNA as genetic material and are found predominantly in humans, other mammals, and birds. There are primarily six coronavirus species known to cause different types of respiratory, neurological, and hepatic diseases [8, 9]. Out of six, four species NL63, OC43, HKU1, and 229E predominantly infect humans and usually cause moderate respiratory tract illness such as common cold [10]. The two other strains which cause severe acute respiratory syndrome (SARS-CoV) and Middle East Respiratory Syndrome (MERS-CoV) are zoonotic in origin and have been related to deadly infections [11,12,13,14]. SARS-CoV was the causal agent of the severe acute respiratory syndrome outbreaks in 2002 and 2003 in Guangdong Province, China [14,13,14,17].
Although so far there are controversies about the source of the COVID-19 and its intermediate host, the evolutionary analysis showed that the coronavirus was most similar to bat coronavirus isolate RaTG13 (GenBank No. MN996532). It has a linear single-stranded RNA genome ~ 30 kb [5, 7, 18] being 96.2% homologous to the nucleotides in the whole genome of the same. Also, it is interesting to note that sequences from the number of recent patients are strikingly identical and show 79.6% sequence identity to SARS-CoV [7, 18, 19]. Sequence alignment of 2019-nCoV, RaTG13, and SARS-CoV showed no proof of any kind of recombination in the genome of nCoV [19].
Along with flu and respiratory problems, people of all ages are susceptible to COVID-19, and there was no significant gender bias in terms of viral infection [2]. Recent researches have revealed that COVID-19 is less lethal in children than adult patients, though some infants were vulnerable to infection [20]. Also, prominent changes were seen in the blood coagulation of patients with SARS-CoV-2 infection. The coagulation function in patients with SARS-CoV-2 is significantly unstable in comparison to healthy people [21]. It is also reported that the patients with pre-existing cardiovascular metabolic diseases such as hypertension, cardia-cerebrovascular diseases, and diabetes are at a greater risk of developing severe conditions and these pre-existing ailments can also greatly affect the prognosis of the COVID-19 [22].
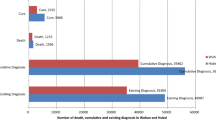
WHO on March 11, 2020 declared COVID-19 a pandemic, pointing to over 118,000 cases of the coronavirus illness in over 110 countries and territories around the world and the sustained risk of further global spread [23]. From December 12, 2019, when the first patient was admitted to hospital, to June 8, 2020, there are 6,931,000 confirmed cases and 400,857 confirmed deaths in more than 200 countries. European region with 2,286,560 positive cases and 184,120 deaths and regions of America with 3,311,387 positive cases and 181,804 deaths are the most affected regions worldwide. In Europe, the UK is the worst affected country with 40,542 deaths to date, followed by Italy, Spain, France, and Germany [24, 25].
Genetics and Molecular Structure of nCoV and Previous Coronaviruses
The virus genome of nCoV consists of six major open reading frames (ORFs) like all other coronaviruses. The 3′-end of ORF1a, the ORF1b, and most of the spike regions of nCoV and RaTG13 were similar to a distant lineage within the Sarbecovirus branch. In this region, nCoV and RaTG13 are distantly related to the bat-SARS-like coronavirus sequences which were also confirmed by phylogenetic analyses, emphasizing that nCoV may have originated in bats [19, 26]. nCoV is distinct from SARS-CoV in terms of the phylogeny of the complete RNA-dependent RNA polymerase (RdRP) gene, reiterating that it is a novel coronavirus from the subgenus Sarbecovirus. The 3-D structure of nCoV revealed that the nCoV receptor-binding domain, composed of a core and an external subdomain which is similar to that of SARS-CoV suggesting that nCoV might as well be thriving by using angiotensin-converting enzyme 2 (ACE2) as a cell receptor. Interestingly, several key residues responsible for the binding of the SARS-CoV receptor-binding domain to the ACE2 receptor were variable in the nCoV receptor-binding domain (Asn439, Asn501, Gln493, Gly485, and Phe486). Past reports confirm that coronavirus contains four key structural proteins: spike (S), membrane (M), envelope (E), and nucleocapsid (N). These are encoded in the 3′-end of the genome (Fig. 1). The S protein utilizes an N-terminal signal sequence to gain access to the endoplasmic reticulum (ER) [27, 28]. The M protein is the most abundant one with 3 transmembrane domains which gives the shape to virion [29,28,31]. The E protein is the least abundant one and is highly divergent. It is known for facilitating the assembly and release of the virus [32]. The N protein is the only protein found in the nucleocapsid of virus particles and binds the viral genome in a beads-on-a-string type conformation. N protein is also reported to bind to replicate complex and the M protein, and these protein interactions tether the viral genome to the replicase-transcriptase complex and package the encapsulated genome into viral particles [33,32,33,34,35,38].
The S gene coding for receptor-binding spike protein which is crucial for membrane fusion and transmission capacity is not very similar to pre-existing CoVs, nonetheless having an exceptional similarity to RaTG13 (93.1%) [39,38,41]. In nCoV, there are three novel insertions in the N-terminal domain and key residues in the receptor-binding motif which are not present in SARS-CoV [7] and possibly, these insertions have a pivotal role in conferring sialic acid-binding activity which is the key factor in determining the pathogenicity of coronaviruses [7, 42,41,44].
First-Line Therapeutics Devised from Existing Antiviral Drugs
The first step in the diagnostic and therapeutic approach is the detection and distinction of nCoV from other CoV strains. Therefore, timely detection of nCoV infection cases is very important to stop the community spread. Real-time PCR (RT-PCR) has been deployed in diagnostic virology as a powerful weapon. Conventionally, the preferred targets of coronavirus RT-PCR assays include the conserved and abundantly expressed genes such as the structural S, E, and N genes; the non-structural RdRP; and replicase open reading frame (ORF) 1a/b genes [45, 46]. Until now, E gene and N gene assays are being used as the first-line screening and RdRP gene assay is being used as a confirmatory test. The RdRP assay is highly sensitive and specific [7, 47,46,49].
Till now, there are no specific US Food and Drug Administration, USA (FDA)-approved drugs for the treatment of nCoV patients. At present, treatments and control measures for severely ill patients include intensive care, supplementary oxygen, and mechanical ventilatory support. Several new drugs are being studied in more than a hundred clinical trials across the globe. FDA issued an emergency use authorization for the investigational antiviral drug remdesivir and antimalarial drug hydroxychloroquine for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease, though the mechanism of actions is still unknown, they showed improved recovery in some severely ill patients. Remdesivir is administered intravenously. It has a broad antiviral activity and is known to inhibit viral replication through premature termination of RNA transcription. There are reports of its in vitro activity against SARS-CoV-2 and in vitro and in vivo activity against beta coronaviruses [50, 51]. One other combination of drugs which has been tested in recent months is lopinavir-ritonavir, but it did not show promise for the treatment of hospitalized COVID-19 patients with pneumonia in a recent clinical trial in China [52].
Recently, hydroxychloroquine and chloroquine had been reported to be of importance in relevance to COVID-19. These are oral prescription drugs that have been used for the treatment of malaria and certain inflammatory conditions like rheumatoid arthritis, systemic lupus erythematosus, and porphyria cutanea tarda. Both of these drugs have in vitro activity against SARS-CoV, SARS-CoV-2, and other coronaviruses, with hydroxychloroquine having relatively higher potency against SARS-CoV-2 [51, 53, 54]. A study in China reported that chloroquine treatment of COVID-19 patients had the clinical and virologic benefit and they recommended this as an antiviral drug or the treatment of COVID-19 in China [55]. In the USA, several clinical trials of hydroxychloroquine for prophylaxis or treatment of SARS-CoV-2 infection are going on. Although its temporary use on patients in hospitals during the COVID-19 pandemic has been authorized by the FDA, still it has not been certified as safe and successful for treating or preventing COVID-19.
One therapy, which is giving a positive hope in the current crisis is plasma therapy. In this therapy, the blood is collected from patients who have recovered from COVID-19 and plasma is separated from that. Convalescent plasma which contains antibodies to nCoV is then administered to patients with COVID-19. The use of convalescent plasma has been studied in previous epidemics like the SARS-CoV-1 epidemic, the H1N1 pandemic, and the MERS-CoV epidemic. This treatment has its roots from the first time use of convalescent plasma for the cure of diphtheria in 1901 by Nobel Prize winner (Physiology and Medicine) Emil von Behring. A number of clinical trials with convalescent plasma are going on worldwide with some showing promising results for the prophylaxis of severe cases of COVID-19 (USA Food & Drug Administration https://www.fda.gov/media/136470/download).
Ongoing Approach and Targets Under Investigation
We have been under the continuous invasion of deadly virus infections like SARS-CoV, Ebola, Nipah, and Zika in recent years. This year, the aggressive outbreak of nCoV has emerged as a brutal pandemic taking the lives of thousands. The repeated surfacing of coronaviruses at repeated intervals is a grave threat to health and economy.
The major concern here is that after decades of research on coronaviruses, there is no first-line defined defense therapeutics or vaccines. This potentiates the utmost need for developing potent vaccines or prophylaxis drugs to put a full stop to the possibility of future outbreaks. Several clinical, genetic, and epidemiological features of nCoV resemble SARS-CoV infection. Hence, the research advancements on SARS-CoV treatment might help the scientific community in a quick understanding of this virus pathogenesis and develop effective prophylactic drugs to treat and prevent this infection.
Some potential drug targets which could be crucial in therapeutic design to prevent COVID-19:
The Spike Protein
The spike (S) protein from coronaviruses has been reported to be a crucial viral antigen which stimulates a strong humoral and cell-mediated immune response in humans. It is also known to mediate receptor binding and virus entry during host infection [14]. The role of S protein in receptor binding and membrane fusion marks it a perfect target for vaccine and therapeutic development. Previous studies on SARS-CoV reveal that vaccines based on the S protein can induce antibodies to block virus binding and fusion or neutralize virus infection [56]. Although to date, there are no certified vaccines available against prior coronaviruses [57, 58]. Since there is a similarity between S protein of MERS-CoV and nCoV, it would be great to target nCoV S protein for therapeutic research. Also, MERS-CoV relies on the binding between its RBD region and the hCD26 receptor to initiate infection [56, 59, 60], interrupting this interaction could abrogate virus invasion and hence could be a great prospective site for drug design.
ACE2
Protein structure modeling studies have shown that (ACE2) is the receptor for the S protein of SARS-CoV. The complex structure of ACE2 with the RBD of S protein of SARS viruses has been resolved and it demonstrated tight interaction among them. Recent studies have confirmed that nCoV binds to ACE2. Structural analysis of potential interactions between RBD of S protein from the human SARS virus and ACE2 protein depicted several interaction points [61, 62]. Interestingly, the interaction between RBD of 2019-nCoV and human ACE2 depicted only one potential hydrophobic interaction between ACE2 (L79, M82) and RBD (F486) and one cation-π interaction ACE2 (K353)/RBD (Y492) (N Dong - 2020 F1000). This gives definitive leads that ACE2 has a pivotal role in virus infectivity and pathogenicity and opens up doors for specific and targeted drug design for combating the pathogenicity and lethality of the nCoV which elicited worldwide pandemic.
GRP78
A spike binding site, host cell receptor glucose-regulated protein 78 (GRP78) which is specific to nCoV has been predicted using docking and bioinformatics analysis. Upon stress, GRP78 is known to act as a chaperone for the misfolded proteins and escape ER to translocate to the cell membrane. It can mediate virus recognitions and entry in the cell by its substrate-binding domain [63,62,65]. GRP78 should bind to nCoV, as it does in the case of the MERS-CoV coronavirus. Four regions of the spike protein were predicted to be the binding site to GRP78, based on sequence and structural similarity [66]. Inhibiting the interaction between GRP78 and nCoV spike protein would probably decrease the rate of viral infection and could be a strong target for therapeutic designs. Also, vaccine designed against the nCoV spike protein could be a potent preventive measure to bring down the mortality rate due to COVID-19.
Sialic Acid-Binding Residues
There are reports which show sialic acid serves as a ligand for the binding of a transmissible gastroenteritis virus (TGEV), which is an enteropathogenic porcine coronavirus to erythrocytes [67, 68]. Sialic acid-binding activity may increase the stability of the virions by the binding of the virus to sialoglycoconjugates. Increased stability would help virions to survive the passage through the gastrointestinal tract [42]. In nCoV, there are three novel insertions in the N-terminal domain unlike that of SARS-CoV [7]. These insertions are thought to confer sialic acid-binding activity and could be very potent targets for future vaccines. Also, sialic acid-binding activity is sensitive to certain competitive inhibitors which could be a good start point for further research and therapeutic targeting [43, 69].
Conclusion
Control measures for COVID-19 are majorly based on the understanding of previously existing coronaviruses, mode of their infections, and consideration of potential modes of transmission. Many labs are working hard in collaboration for the development of therapeutics and vaccines to put a full stop to thousands of deaths occurring worldwide every day. Few of them have cleared phase I trials and there is an anticipation of a potential vaccine by the end of the year 2020. Clinicians are advised to see the Centers for Disease Control and Prevention (CDC), USA, website for changes in guidance for testing, treatment, and infection control. Response actions against the ferocious nCoV must be aggressive. Until a new vaccine against nCoV becomes available, avoidance of viral spreading is the most appropriate way to flatten the curve for the number of people getting infected and dying due to COVID-19.
References
Su YJ, Lai YC. Comparison of clinical characteristics of coronavirus disease (COVID-19) and severe acute respiratory syndrome (SARS) as experienced in Taiwan. Travel Med Infect Dis. 2020:101625. https://doi.org/10.1016/j.tmaid.2020.101625.
WHO Situation Report –22 (2020) Novel Coronavirus (2019-nCoV). Available at:https://reader.elsevier.com/reader/sd/pii/S1201971220300114?token=97F6544AD7B289380CED1754D60197EE3F8E62D33CDF8964C4AA3B047348B0E5F5D84D263B13377329872C81AD6D7859
Ceraolo C, Giorgi FM. Genomic variance of the 2019-nCoV coronavirus. J Med Virol. 2020;92:522–8.
Lai CC, Liu YH, Wang CY, Wang YH, Hsueh SC, Yen MY, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect. 2020. https://doi.org/10.1016/j.jmii.2020.02.012.
Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–74.
Shi Z, Hu Z. A review of studies on animal reservoirs of the SARS coronavirus. Virus Res. 2008;133:74–87.
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3.
Masters PS. The molecular biology of coronaviruses. Adv Virus Res. 2006;66:193–292.
Weiss SR, Leibowitz JL. Coronavirus pathogenesis. Adv Virus Res. 2011;81:85–164.
Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, et al. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016;24:490–502.
de Wilde AH, Jochmans D, Posthuma CC, Zevenhoven-Dobbe JC, van Nieuwkoop S, Bestebroer TM, et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58:4875–84.
de Wit E, Rasmussen AL, Falzarano D, Bushmaker T, Feldmann F, Brining DL, et al. Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc Natl Acad Sci U S A. 2013;110:16598–16,603.
Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–92.
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33.
Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, Becker S, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–76.
Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–66.
Zhong NS, Zheng BJ, Li YM, Poon Z, Xie H, Chan KH, et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet. 2003;362:1353–8.
Li X, Zai J, Zhao Q, Nie Q, Li Y, Foley BT, et al. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J Med Virol. 2020b. https://doi.org/10.1002/jmv.25731.
Paraskevis D, Kostaki EG, Magiorkinis G, Panayiotakopoulos G, Sourvinos G, Tsiodras S. Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect Genet Evol. 2020;79:104212.
Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020. https://doi.org/10.1542/peds.2020-0702.
Han H, Yang L, Liu R, Liu F, Wu KL, Li J, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020. https://doi.org/10.1515/cclm-2020-0188.
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020a. https://doi.org/10.1007/s00392-020-01626-9.
WHO Situation Report –51 (2020) Coronavirus disease 2019 (COVID-19). Available at:https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10
Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–60.
WHO Situation Report −140 (2020) Coronavirus disease 2019 (COVID-19). Available https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200608-covid-19-sitrep-140.pdf?sfvrsn=2f310900_2
Luk HKH, Li X, Fung J, Lau SKP, Woo PCY. Molecular epidemiology, evolution and phylogeny of SARS coronavirus. Infect Genet Evol. 2019;71:21–30.
Beniac DR, Andonov A, Grudeski E, Booth TF. Architecture of the SARS coronavirus prefusion spike. Nat Struct Mol Biol. 2006;13:751–2.
Delmas B, Laude H. Assembly of coronavirus spike protein into trimers and its role in epitope expression. J Virol. 1990;64:5367–75.
Armstrong J, Niemann H, Smeekens S, Rottier P, Warren G. Sequence and topology of a model intracellular membrane protein, E1 glycoprotein, from a coronavirus. Nature. 1984;308:751–2.
Godet M, L’Haridon R, Vautherot JF, Laude H. TGEV corona virus ORF4 encodes a membrane protein that is incorporated into virions. Virology. 1992;188:666–75.
Neuman BW, Kiss G, Kunding AH, Bhella D, Baksh MF, Connelly S, et al. A structural analysis of M protein in coronavirus assembly and morphology. J Struct Biol. 2011;174:11–22.
DeDiego ML, Alvarez E, Almazan F, Rejas MT, Lamirande E, Roberts A, et al. A severe acute respiratory syndrome coronavirus that lacks the E gene is attenuated in vitro and in vivo. J Virol. 2007;81:1701–13.
Chang CK, Sue SC, Yu TH, Hsieh CM, Tsai CK, Chiang YC, et al. Modular organization of SARS coronavirus nucleocapsid protein. J Biomed Sci. 2006;13:59–72.
Hurst KR, Koetzner CA, Masters PS. Identification of in vivo-interacting domains of the murine coronavirus nucleocapsid protein. J Virol. 2009;83:7221–34.
Kuo L, Masters PS. Functional analysis of the murine coronavirus genomic RNA packaging signal. J Virol. 2013;87:5182–92.
Molenkamp R, Spaan WJ. Identification of a specific interaction between the coronavirus mouse hepatitis virus A59 nucleocapsid protein and packaging signal. Virology. 1997;239:78–86.
Nal B, Chan C, Kien F, Siu L, Tse J, Chu K, et al. Differential maturation and subcellular localization of severe acute respiratory syndrome coronavirus surface proteins S, M and E. J Gen Virol. 2005;86:1423–34.
Sturman LS, Holmes KV, Behnke J. Isolation of coronavirus envelope glycoproteins and interaction with the viral nucleocapsid. J Virol. 1980;33:449–62.
Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3:237–61.
Lu G, Wang Q, Gao GF. Bat-to-human: spike features determining ‘host jump’ of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol. 2015;23:468–78.
Wang Q, Wong G, Lu G, Yan J, Gao GF. MERS-CoV spike protein: targets for vaccines and therapeutics. Antivir Res. 2016;133:165–77.
Krempl C, Schultze B, Herrler G. Analysis of cellular receptors for human coronavirus OC43. Adv Exp Med Biol. 1995;380:371–4.
Schwegmann-Wessels C, Bauer S, Winter C, Enjuanes L, Laude H, Herrler G. The sialic acid binding activity of the S protein facilitates infection by porcine transmissible gastroenteritis coronavirus. Virol J. 2011;8:435.
Schwegmann-Wessels C, Herrler G. Sialic acids as receptor determinants for coronaviruses. Glycoconj J. 2006;23:51–8.
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13.
Mackay IM, Arden KE. MERS coronavirus: diagnostics, epidemiology and transmission. Virol J. 2015;12:222.
Chan JF, Yip CC, To KK, Tang TH, Wong SC, Leung KH, et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-polymerase chain reaction assay validated in vitro and with clinical specimens. J Clin Microbiol. 2020. https://doi.org/10.1128/JCM.00310-20.
Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25. https://doi.org/10.2807/1560-7917.ES.2020.25.3.2000045
Reusken C, Broberg EK, Haagmans B, Meijer A, Corman VM, Papa A, et al. Laboratory readiness and response for novel coronavirus (2019-nCoV) in expert laboratories in 30 EU/EEA countries, January 2020. Euro Surveill. 2020;25. https://doi.org/10.2807/1560-7917.ES.2020.25.6.2000082
Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9. https://doi.org/10.1126/scitranslmed.aal3653
Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–71.
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020. https://doi.org/10.1056/NEJMoa2001282.
Colson P, Rolain JM, Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J Antimicrob Agents. 2020;55:105923.
Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, et al. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009.
Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–3.
Du L, Yang Y, Zhou Y, Lu L, Li F, Jiang S. MERS-CoV spike protein: a key target for antivirals. Expert Opin Ther Targets. 2017;21:131–43.
Papaneri AB, Johnson RF, Wada J, Bollinger L, Jahrling PB, Kuhn JH. Middle East respiratory syndrome: obstacles and prospects for vaccine development. Expert Rev Vaccines. 2015;14:949–62.
Zhang N, Tang J, Lu L, Jiang S, Du L. Receptor-binding domain-based subunit vaccines against MERS-CoV. Virus Res. 2015;202:151–9.
Du Z, Wang L, Cauchemez S, Xu X, Wang X, Cowling BJ, et al. Risk for transportation of 2019 novel coronavirus disease from Wuhan to other cities in China. Emerg Infect Dis. 2020a;26. https://doi.org/10.3201/eid2605.200146
Du Z, Xu X, Wu Y, Wang L, Cowling BJ, Meyers LA. Serial interval of COVID-19 among publicly reported confirmed cases. Emerg Infect Dis. 2020b;26. https://doi.org/10.3201/eid2606.200357.
Li F, Li W, Farzan M, Harrison SC. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005a;309:1864–8.
Li W, Shi Z, Yu M, Ren W, Smith C, Epstein JH, et al. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005b;310:676–9.
Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373–81.
Quinones QJ, de Ridder GG, Pizzo SV. GRP78: a chaperone with diverse roles beyond the endoplasmic reticulum. Histol Histopathol. 2008;23:1409–16.
Rao RV, Peel A, Logvinova A, del Rio G, Hermel E, Yokota T, et al. Coupling endoplasmic reticulum stress to the cell death program: role of the ER chaperone GRP78. FEBS Lett. 2002;514:122–8.
Ibrahim IM, Abdelmalek DH, Elshahat ME, Elfiky AA. COVID-19 spike-host cell receptor GRP78 binding site prediction. J Infect. 2020. https://doi.org/10.1016/j.jinf.2020.02.026.
Pensaert M, Cox E, van Deun K, Callebaut P. A sero-epizootiological study of porcine respiratory coronavirus in Belgian swine. Vet Q. 1993;15:16–20.
Schultze B, Enjuanes L, Herrler G. Analysis of the sialic acid-binding activity of the transmissible gastroenteritis virus. Adv Exp Med Biol. 1995;380:367–70.
Herrler G, Gross HJ, Brossmer R. A synthetic sialic acid analog that is resistant to the receptor-destroying enzyme can be used by influenza C virus as a receptor determinant for infection of cells. Biochem Biophys Res Commun. 1995;216:821–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
No patient or clinical sample used in this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on COVID-19
Rights and permissions
About this article
Cite this article
Singh, A.B., Singh, N. Novel Coronavirus (nCoV): a Bitter Old Enemy in a New Avatar. SN Compr. Clin. Med. 2, 1083–1088 (2020). https://doi.org/10.1007/s42399-020-00373-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42399-020-00373-2