Abstract
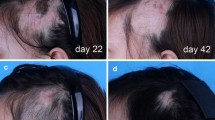
Radiation-induced alopecia is very rare in neurointerventional surgery due to limited radiation dose. Furthermore, no case referred to the reversible hair loss after angioplasty rather than endovascular embolization. A 54-year-old man with diabetes manifested a sign of alopecia after an endovascular stenting for symptomatic basilar artery stenosis. The topography of hair loss patch was in a shape of a rectangle, and it recovered literally in 6 months, without any treatment. The reduction of exposure of patients to radiation during fluoroscopically directed interventional procedures should be performed as possible. Speculatively, metabolic abnormalities such as diabetes and obesity may be considered as an underlying risk that induces skin manifestation in a process of exposure to radiation.

Similar content being viewed by others
References
Tosti A, Piraccini BM, Alagna G. Temporary hair loss simulating alopecia areata after endovascular surgery of cerebral arteriovenous malformations: a report of 3 cases [J]. Arch Dermatol. 1999;135(12):1555–6.
Wen CS, Lin SM, Chen Y, Chen JC, Wang YH, Tseng SH. Radiation-induced temporary alopecia after embolization of cerebral arteriovenous malformations [J]. Clin Neurol Neurosurg. 2003;105(3):215–7.
Marti N, Lopez V, Pereda C, Martin JM, Montesinos E, Jorda E. Radiation-induced temporary alopecia after embolization of cerebral aneurysms [J]. Dermatol Online J. 2008;14(7):19.
Foroozan M, Bracard S, Schmutz JL. Alopecia after cerebral endovascular procedure [J]. J Eur Acad Dermatol Venereol. 2009;23(5):573–4. https://doi.org/10.1111/j.1468-3083.2008.02957.x.
Vano E, Fernandez JM, Sanchez RM, Martinez D, Ibor LL, Gil A, et al. Patient radiation dose management in the follow-up of potential skin injuries in neuroradiology [J]. AJNR Am J Neuroradiol. 2013;34(2):277–82. https://doi.org/10.3174/ajnr.A3211.
Derdeyn CP, Chimowitz MI, Lynn MJ, Fiorella D, Turan TN, Janis LS, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial [J]. Lancet. 2014;383(9914):333–41. https://doi.org/10.1016/S0140-6736(13)62038-3.
Jiang WJ, Yu W, Du B, et al. Outcome of patients with >/=70% symptomatic intracranial stenosis after wingspan stenting [J]. Stroke. 2011;42(7):1971–5. https://doi.org/10.1161/STROKEAHA.110.595926.
Yu SC, Leung TW, Lee KT, et al. Angioplasty and stenting of intracranial atherosclerosis with the wingspan system: 1-year clinical and radiological outcome in a single Asian center [J]. J Neurointerv Surg. 2014;6(2):96–102. https://doi.org/10.1136/neurintsurg-2012-010608.
Hussain M, Datta N, Cheng Z, et al. Spanning from the West to East: an updated review on endovascular treatment of intracranial atherosclerotic disease [J]. Aging Dis. 2017;8(2):196–202. https://doi.org/10.14336/AD.2016.0807.
Lawenda BD, Gagne HM, Gierga DP, Niemierko A, Wong WM, Tarbell NJ, et al. Permanent alopecia after cranial irradiation: dose-response relationship [J]. Int J Radiat Oncol Biol Phys. 2004;60(3):879–87. https://doi.org/10.1016/j.ijrobp.2004.04.031.
Gonzalez-Saldivar G, Rodriguez-Gutierrez R, Ocampo-Candiani J, et al. Skin manifestations of insulin resistance: from a biochemical stance to a clinical diagnosis and management [J]. Dermatol Ther (Heidelb). 2017;7(1):37–51. https://doi.org/10.1007/s13555-016-0160-3.
Stefanadi EC, Dimitrakakis G, Antoniou CK, Challoumas D, Punjabi N, Dimitrakaki IA, et al. Metabolic syndrome and the skin: a more than superficial association. Reviewing the association between skin diseases and metabolic syndrome and a clinical decision algorithm for high risk patients [J]. Diabetol Metab Syndr. 2018;10:9. https://doi.org/10.1186/s13098-018-0311-z.
Valentin J. Avoidance of radiation injuries from medical interventional procedures [J]. Ann ICRP. 2000;30(2):7–67. https://doi.org/10.1016/S0146-6453(01)00004-5.
Availability of Data and Materials
The datasets supporting the conclusions of this article are included within the article and its additional files.
Author information
Authors and Affiliations
Contributions
Peng Zhang analyzed and interpreted the case, collected the clinical data, and drafted the manuscript. Wen-Hua Fu participated in the design of the case report. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that they have no competing interests.
Ethics Approval and Consent to Participate
This case report involves a patient. The case report complies with the Helsinki Declaration, and approval was obtained from the ethics committee of PLA Joint Logistics Support Force No.988 Hospital.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editor of this journal.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Rights and permissions
About this article
Cite this article
Zhang, P., Fu, WH. Reversible Localized Alopecia After Angioplasty of Basilar Artery Stenosis in a Diabetic Patient: a Case Report. SN Compr. Clin. Med. 2, 992–994 (2020). https://doi.org/10.1007/s42399-020-00354-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42399-020-00354-5