Abstract
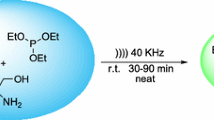
The activation of Baylis–Hillman reaction by aliquat-336 and triphenylphosphine under ultrasound radiation is described. A significant improvement of the yield of Baylis–Hillman reaction was observed. We illustrate the effects of the solvent, the nature of the nucleophilic agent, the phase transfer catalyst and ultrasound, on the Baylis–Hillman reaction efficiency. The reaction examined involves methyl acrylate, which, in the presence of nucleophilic agent, forms a zwitterionic intermediate, which reacts with the aldehyde to subsequently give the product of the reaction. For all the aldehydes tested, it was found that ultrasound radiation in the presence of aliquat-336 could reduce the duration of the Baylis–Hillman reaction and improve the reaction efficiency. This method could be a clean, economical, efficient and safe technology in organic synthesis to prepare Baylis–Hillman adducts.


Similar content being viewed by others
References
Xie P, Huang Y (2015) Org Biomol Chem 13:8578–8595
Barbier V, Couty F, David ORP (2015) Eur J Org Chem 2015:3679–3688
Zeng XH, Wang HM, Ding MW (2015) Org Lett 17:2234–2237
Chen J, Li J, Wang J, Li H, Wang W, Guo Y (2015) Org Lett 17:2214–2217
Basavaiah D, Reddy BS, Badsara SS (2010) Chem Rev 110:5447–5674
Aggarwal VK, Dean DK, Mereu A, Williams R (2002) J Org Chem 67:510–514
Basavaiah D, Rao AJ, Satyanarayana T (2003) Chem Rev 103:811–891
Basavaiah D, Rao KV, Reddy RJ (2007) Chem Soc Rev 36:1581–1588
Basavaiah D, Rao PD, Hyma RS (1996) Tetrahedron 52:8001–8062
Loh TP, Cao GQ, Pei J (1998) Tetrahedron Lett 39:1457–1460
Wang B, Yu XM, Lin GQ (2001) Synlett 2001:904–906
Anand RV, Baktharaman S, Singh VK (2002) Tetrahedron Lett 43:5393–5395
Li CJ (2005) Chem Rev 105:3095–3165
Moussaoui Y, Ben Salem R (2003) J Tun Chem Soc 5:255–263
Moussaoui Y, Ben Salem R (2007) C R Chimie 10:630–636
Cravotto G, Borretto E, Oliverio M, Procopio A, Penoni A (2015) Catal Commun 63:2–9
Saïd K, Moussaoui Y, Kammoun M, Ben Salem R (2011) Ultrason Sonochem 18:23–27
Mhamdi L, Said K, Rigane G, Ben Salem R, Moussaoui Y (2016) J Tun Chem Soc 18:61–67
Mhamdi L, Saïd K, Moussaoui Y, Ben Salem R (2013) J Soc Chem Tunisie 15:149–162
Said K, Ben Salem R (2016) Adv Chem Eng Sci 6:111–123
Cravotto G, Beggiato M, Penoni A, Palmisano G, Tollari S, Leveque JM, Bonrath W (2005) Tetrahedron Lett 46:2267–2271
Cella R, Stefani HA (2006) Tetrahedron 62:5656–5662
Pattarawarapan M, Jaita S, Wangngae S, Phakhodee W (2016) Tetrahedron Lett 57:1354–1358
Song YL, Dong YF, Wu F, Yang T, Yang GL (2015) Ultrason Sonochem 22:119–124
Banerjee B (2017) Ultrason Sonochem 35:1–14
Banerjee B (2017) Ultrason Sonochem 35:15–35
Ge SQ, Hua YY, Xia M (2009) Ultrason Sonochem 16:232–236
Lorimer JP, Mason TJ (1987) Chem Soc Rev 16:239–274
Datta B, Pasha MA (2012) Ultrason Sonochem 19:725–728
Chen BH, Li JT, Chen GF (2015) Ultrason Sonochem 23:59–65
Dupuy C, Petrier C, Sarandeses LA, Luche JL (1991) Synth Commun 21:643–651
Dange PN, Kulkarni AV, Rathod VK (2015) Ultrason Sonochem 26:257–264
Harikumar K, Rajendran V (2015) Inter J Chem Sci Appl 6:12–28
Sancheti SV, Gogate PR (2017) Ultrason Sonochem 36:527–543
Alonso F, Beletskaya IP, Yus M (2005) Tetrahedron 61:11771–11835
Margulis MA (2004) High Energ Chem 38:135–142
Singh BS, Lobo HR, Pinjari DV, Jarag KJ, Pandit AB, Shankarling GS (2013) Ultrason Sonochem 20:633–639
Coelho F, Almeida WP, Veronese D, Mateus CR, Lopes ECS, Rossi RC, Silveira GPC, Pavam CH (2002) Tetrahedron 58:7437–7447
Ge SQ, Hua YY, Xia M (2009) Ultrason Sonochem 16:743–746
Mamaghani M, Dastmard S (2009) Ultrason Sonochem 16:445–447
Yamada YMA, Ikegami S (2000) Tetrahedron Lett 41:2165–2169
Shi M, Xu YM (2002) Eur J Org Chem 2002:696–701
Shi M, Xu YM, Zhao GL, Wu XF (2002) Eur J Org Chem 2002:3666–3679
Shi M, Zhao GL (2002) Tetrahedron Lett 43:4499–4502
Bode ML, Kaye PT (1991) Tetrahedron Lett 32:5611–6514
Acknowledgements
The authors would like to express their profound gratitude to the Tunisian Ministry of Higher Education for its financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mhamdi, L., Said, K., Atoui, D. et al. Baylis–Hillman Reaction Assisted by aliquat-336 and triphenylphosphine Under Ultrasound Radiation. Chemistry Africa 1, 11–16 (2018). https://doi.org/10.1007/s42250-018-0006-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-018-0006-8