Abstract
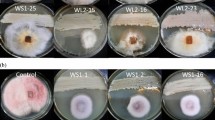
This study aimed at controlling the common fungi causing postharvest Aspergillus rot of cv. Thompson seedless table grape (Vitis vinifera L.) via application of epiphytic biocontrol agents. Antagonistic yeasts and bacteria were isolated from the epiphytic flora associated with grape berries and leaves from five vineyards in Iran. A total of 130 yeast and bacterial isolates from grapevine surfaces were screened for antagonism against Aspergillus flavus, A. niger and A. ochraceus, the main species responsible for the accumulation of aflatoxin and ochratoxin A in grape berries. Seven yeast and bacterial isolates were selected based on their inhibitory effects on Aspergillus spp. and assayed by an in vitro nutritional competition test for their antagonistic capability. These isolates showed obvious antifungal activity against three different species of Aspergillus. Five yeast isolates were identified based on ITS region sequences as Candida membranifasciens (isolates Ka15 and Kh69) and Meyerozyma guilliermondii (Ka21, Kh59 and Kh60). Two bacterial isolates were identified based on the 16S rRNA gene sequences as Bacillus sp. (Ka3 and A10). Finally, the effect of antagonistic isolates on inoculated grape berries for their ability to inhibit infection by Aspergillus spp. was also investigated. All isolates showed antagonistic properties against the pathogens assayed at 25 °C and significantly reduced the disease progress on grape berries. Our data demonstrated that application of antagonistic microorganisms could be a promising alternative to fungicide treatments for controlling postharvest diseases of grapevine.








Similar content being viewed by others
References
Abrunhosa L, Paterson RRM, Venancio A (2010) Biodegradation of ochratoxin a for food and feed decontamination. Toxins 2:1078–1099. https://doi.org/10.3390/toxins2051078
Allam N.G., El-Shanshoury A.R., Emara H.A., Mohamed A.Z., 2008. Fungi, toxigenic fungi, mycotoxins, pathogenic bacteria and heavy metal levels in some medicinal and food herbs. Proceedings. 5 th International Conference of Biological Science (Botany), Tanta (Egypt): 29–39
Allam NG, El-Shanshoury AR, Emara HA, Zaky AZ (2012) Decontamination of ochratoxin a producing Aspergillus niger and ochratoxin a in medicinal plants by gamma irradiation and essential oils. Journal of International Environmental Application and. Science 7:161–169
Arras G (1996) Mode of action of an isolate of Candida famata in biological control of Penicillium digitatum in orange fruit. Postharvest Biol Technol 8:191–198
Arrebola E, Sivakumar D, Korsten L (2010) Effect of volatile compounds produced by Bacillus strains on postharvest decay in citrus. Biol Control 53:122–128
Bae S, Fleet GH, Heard GM (2004) Occurrence and significance of Bacillus thuringiensis on wine grapes. Int J Food Microbiol 94:301–312
Batta YA (2000) Alternaria leaf spot disease on fig trees: varietal susceptibility and effect of some fungicides and Trichoderma. The Islamic University of Gaza. Journal 8:83–97
Batta YA (2001) Effect of fungicides and antagonistic microorganisms on the black fruit spot disease on persimmon., Dirasat. Agric Sci 28:165–171
Batta YA (2004a) Postharvest biological control of apple gray mold by Trichoderma harzianum Rifai formulated in an invert emulsion. Crop Prot 23:19–26
Batta YA (2004b) Effect of treatment with Trichoderma harzianum Rifai formulated in invert emulsion on postharvest decay of apple blue mold. Int J Food Microbiol 96:281–288
Benitez T, Rincon AM, Limon MC, Codon AC (2004) Biocontrol mechanisms of Trichoderma strains. Int Microbiol 7:249–260
Bleve G, Grieco F, Cozzi G, Logrieco A, Visconti A (2006) Isolation of epiphytic yeasts with potential for biocontrol of Aspergillus carbonarius and A. niger on grape. Int J Food Microbiol 108:204–209
Castoria R, de Curtis F, Lima G, Caputo L, Pacifico S, de Cicco V (2001) Aureobasidium pullulans (LS 30), an antagonist of postharvest pathogens of fruits: study on its mode of action. Postharvest Biol Technol 22:7–17
Chalutz E, Ben Arie R, Droby S, Cohen L, Weiss B, Wilson CL (1988) Yeasts as biocontrol agents of postharvest diseases of fruits. Phytoparasitica 16:69–75
Chen H, Xiao X, Wang J, Wu LJ, Zheng ZM, Yu ZL (2008) Antagonistic effects of volatiles generated by Bacillus subtilis on spore germination and hyphal growth of the plant pathogen, Botrytis cinerea. Biotechnol Lett 30:919–923
Csutak O, Vassu T, Sarbu I, Stoica I, Cornea P (2013) Antagonistic activity of three newly isolated yeast strains from the surface of fruits. Food Technol Biotechnol 51:70–77
Diguta CF, Rousseaux S, Weidmann S, Bretin N, Vincent B, Guilloux-Benatier M, Alexandre H (2010) Development of a qPCR assay for specific quantification of Botrytis cinerea on grapes. FEMS Microbiol Lett 313:81–87
Drik R (2000) Specific PCR primers to identify arbuscular mycorrhizal fungi within colonized roots. Mycorrhiza 10:73–80
Droby S, Chalutz E, Wilson CL, Wisniewski M (1989) Characterization of the biocontrol activity of Debaromyces hansenii in the control of Penicillium digitatum on grapefruit. Can J Microbiol 35:794–800
Droby S, Chalutz E, Wilson CL, Wisniewski ME (1992) Biological control of postharvest diseases: a promising alternative to the use of synthetic fungicides. Phytoparasitica 20:S149–S153
Droby S, Wisniewski ME, Cohen L, Weiss B, Touitou D, Eilam Y, Chalutz E (1997) Influence of CaCl2 on Penicillium digitatum, grapefruit peel tissue, and biocontrol activity of Pichia guilliermondii. Phytopathology 87:310–315
Droby S., Wilson C.L., Wisniewski M., El Ghaouth A., 2000. Biologically based technology for the control of postharvest diseases of fruits and vegetables. In: Wilson C.L., Droby S. (eds). Microbial food contamination, pp 187–205. CRC Press, Boca Raton
Eckert JW, Ogawa JM (1988) The chemical control of postharvest diseases: deciduous fruit, berries, vegetables and root tuber crops. Annu Rev Phytopathol 26:433–469
Elad Y, Kirshner B (1993) Survival in the phylloplane of an introduced biocontrol agent (Trichoderma harzianum) and populations of the plant pathogen Botrytis cinerea as modified by biotic conditions. Phytoparasitica 21:303–313
El-Ghaouth A, Wilson CL, Wisniewski M (1998) Ultrastructural and cytochemical aspects of the biological control of Botrytis cinerea by Candida saitoana in apple fruit. Phytopathology 88:282–291
Filonow AB, Vishniac HS, Anderson JA, Janisiewicz WJ (1996) Biological control of Botrytis cinerea in apple by yeasts from various habitats and their putative mechanism of antagonism. Biol Control 7:212–220
Foldes T, Banhegyi I, Herpai Z, Varga L, Szigeti J (2000) Isolation of Bacillus strains from the rhizosphere of cereals and in vitro screening for antagonism against phytopathogenic, food-borne pathogenic and spoilage microorganisms. J Appl Microbiol 89:840–846
Gabriolotto C, Monchiero M, Negre M, Spadaro D, Gullino ML (2009) Effectiveness of control strategies against Botrytis cinerea in vineyard and evaluation of the residual fungicide concentrations. J Environ Sci Health B 44:389–396
Gachomo EW, Kotchoni SO (2008) The use of Trichoderma harzianum and T. viride as potential biocontrol agents against peanut microflora and their effectiveness in reducing aflatoxin contamination of infected kernels. Biotechnology 7:439–447
Harman GE, Howell Ch R, Viterbo A, Chet I, Matteo L (2004) Trichoderma species opportunistic, a virulent plant symbionts. Nat Rev Microbiol 2:43–56
Janisiewicz WJ, Roitman J (1988) Biological control of blue mold and gray mold on apple and pear with Pseudomonas cepacia. Postharvest Pathology and Mycotoxins 78:1697–1700
Jiang C, Shi J, Liu Y, Zhu C (2014) Inhibition of Aspergillus carbonarius and fungal contamination in table grapes using Bacillus subtilis. Food Control 35:41–48
Karabulut OA, Smilanic JL, Gabler FM, Mansour M, Droby S (2003) Near-harvest applications of Metschnikowia fructicola, ethanol, and sodium bicarbonate to control postharvest diseases of grape in Central California. Plant Dis 87:1384–1389
Kim DS, Cook RJ, Weller DM (1997) Bacillus sp. L324-92 for biological control of three root diseases of wheat grown with reduced tillage. Phytopathology 87:551–558
Klich MA, Lax AR, Bland JM (1991) Inhibition of some mycotoxigenic fungi by iturin a, a peptidolipid produced by Bacillus subtilis. Mycopathologia 116:77–80
Kraus J, Lopper JE (1990) Biocontrol of Pythium damping-off of cucumber by Pseudomonas fluorescens pf-5: mechanistic studies. The Second International Workshop on Plant Growth-Promoting Rhizobacteria, Interlaken, pp 172–175
Leelasuphakul W, Hemmaneea P, Chuenchitt S (2008) Growth inhibitory properties of Bacillus subtilis strains and their metabolites against the green mold pathogen (Penicillium digitatum Sacc.) of citrus fruit. Postharvest Biol Technol 48:113–121
Liu HB, Tian SP, Qin GZ, Xu Y (2002) Effect of Cryptococcus laurentii on biological control of postharvest diseases in grapes. Agric Sci China 35:831–835
Madden AA, Boyden SD, Soriano JAN, Corey TB, Leff JW, Fierer N, Starks PT (2017) The emerging contribution of social wasps to grape rot disease ecology. Peer Journal 5:e3223
Manici L, Lazzeri L, Palmieri S (1997) In vitro fungitoxic activity of some glucosinolates and their enzyme-derived products toward plant pathogenic fungi. J Agric Food Chem 45:2768–2773
Mari M, Guizzardi M, Pratella GC (1996) Biological control of gray mold in pears by antagonistic bacteria. Biol Control 7:30–37
Masih E, Paul B (2002) Secretion of β-1,3-glucanases by the yeast Pichia membranifaciens and its possible role in the biocontrol of Botrytis cinerea causing grey mold disease of the grapevine. Curr Microbiol 44:391–395
Nally MC, Pescea VM, Maturanoa YP, Toroa ME, Combinab M, Castellanos De Figueroa LI, Vazquez F (2013) Biocontrol of fungi isolated from sour rot infected table grapes by Saccharomyces and other yeast species. Postharvest Biol Technol 86:456–462
Nunes CA (2012) Biological control of postharvest diseases of fruit. Eur J Plant Pathol 133:181–196
O'Neill TM, Elad Y, Shtienberg D, Cohen A (1996) Control of grapevine grey mold with Trichoderma harzianum T39. Biocontrol Sci Tech 6:139–146
Paster N, Droby S, Chalutz E, Menasherov M, Nitzan R, Wilson CL (1993) Evaluation of the potential of the yeast Pichia guilliermondii as a biocontrol agent against Aspergillus flavus and fungi of stored soya beans. Mycol Res 97:1201–1206
Peng G, Sutton JC (1991) Evaluation of microorganisms for biocontrol of Botrytis cinerea in strawberry. Can J Plant Pathol 13:247–257
Pimenta RS, Morais PB, Rosa CA, Correa A (2009) Utilization of yeast in biological control programs. In: Satyanarayana T, Kun G (eds) Yeast biotechnology: diversity and applications. Springer, Berlin, pp 199–214
Poppe L, Vanhoutte S, Hofte M (2003) Mode of action of Pantoea agglomerans CPA-2, an antagonist of postharvest pathogens on fruits. Eur J Plant Pathol 109:963–973
Prusky D (2011) Reduction of the incidence of postharvest quality losses, and future prospects. Food Security 3:463–474
Rabosto X, Carrau M, Paz A, Boido E, Dellacassa E, Carraul FM (2006) Grapes and vineyard soils as sources of microorganisms for biological control of Botrytis cinerea. Am J Enol Vitic 57:332–338
Raspor P, Miklic-Milek D, Avbelj M, Cadez N (2010) Biocontrol of grey mold disease on grape caused by Botrytis cinerea with autochthonous wine yeasts. Food Technol Biotechnol 48:336–343
Romanazzi G, Lichter A, Gabler FM, Smilanick JL (2012) Recent advances on the use of natural and safe alternatives to conventional methods to control postharvest gray mold of table grapes. Postharvest Biol Technol 63:141–147
Sambrook J, Russel DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratoy press. Cold Spring Harbor, New York
Santos A, Marquina D (2004) Killer toxin of Pichia membranifaciens and its possible use as a biopreservative agent to control grey mold disease of grapevine. Microbiology 150:2527–2534
Santos A, Marquina D, Leal JA, Peinado JM (2000) (1,6)-β-D-glucan as cell wall receptor for Pichia membranifaciens killer toxin. Appl Environ Microbiol 66:1809–1813
Schena L, Nigro F, Pentimone I, Ligorio A, Ippolito A (2003) Control of postharvest rots of sweet cherries and table grapes with endophytic isolates of Aureobasidium pullulans. Postharvest Biol Technol 30:209–220
Senthil R, Prabakar K, Rajendran L, Karthikeyan G (2011) Efficacy of different biological control agents against major postharvest pathogens of grapes under room temperature storage conditions. Phytopathol Mediterr 50:55–64
Serra R, Bragab A, Venancio A (2005) Mycotoxin-producing and other fungi isolated from grapes for wine production, with particular emphasis on ochratoxin a. Res Microbiol 156:515–521
Sherman F, Fink G, Hicks J (1986) Methods in yeast genetics. Cold spring harbour laboratory press. Cold Spring Harbor, New York
Stapleton JJ, Grant RS (1992) Leaf removal for nonchemical control of the summer bunch rot complex of wine grapes in the San Joaquin Valley. Plant Dis 76:205–208
Swadling I, Jeffries P (1996) Isolation of microbial antagonists for biocontrol of grey mold disease of strawberries. Biocontrol Sci Tech 6:125–136
Thomidis T, Pantazis S, Konstantinoudis K (2016) Evaluation of serenade max to control fruit rot of grapes. J Agric Sci 8:212–214
Vargas M, Garrido F, Zapata N, Tapia M (2012) Isolation and selection of epiphytic yeast for biocontrol of Botrytis cinerea Pers. on table grapes. Chilean Journal of Agricultural Research 72:332–337
Visconti A, Perrone G, Cozzi G, Solfrizzo M (2008) Managing ochratoxin a risk in the grape-wine food chain. Food Additives and Contaminants-Part A 25:193–220
Wilson CL, Wisniewski ME (1989) Biological control of postharvest diseases of fruit and vegetables: an emerging technology. Annu Rev Phytopathol 27:425–441
Wilson CL, Wisniewski ME (1994) Biological control of postharvest diseases of fruit and vegetables. In: Theory and practice pp 63–75. CRC Press, Boca Raton
Wisniewski M, Wilson CL (1992) Biological control of postharvest diseases of fruits and vegetables: recent advances. Horticulture Science 27:94–98
Wisniewski M., Biles C., Droby S., 1990. The use of the yeast Pichia guilliermondii as a biocontrol agent: characterization of attachment to Botrytis cinerea. Biological Control of Postharvest Diseases of Fruits and Vegetables pp 167–183. Agricultural Research Service document ARS-92. U.S. Department of Agriculture, Washington, D.C,, USA
Yashiro E, Spear RN, McManus PS (2011) Culture-dependent and culture-independent assessment of bacteria in the apple phyllosphere. J Appl Microbiol 110:1284–1296
Zahavi T, Cohen L, Weiss B, Schena L, Daus A, Kaplunov T, Zutkhi J, Ben-Arie R, Droby S (2000) Biological control of Botrytis, Aspergillus and Rhizopus rots on table and wine grapes in Israel. Postharvest Biol Technol 20:115–124
Zhimo VY, Bhutia DD, Saha J (2016) Biological control of postharvest fruit diseases using antagonistic yeasts in India. J Plant Pathol 98:275–283
Zolan M, Pukkila P (1986) Inheritance of DNA methylation in Coprinus cinereus. Mol Cell Biol 6:195–200
Acknowledgements
We thank Ferdowsi University of Mashhad, Iran, for financial support of this research with project number 3/39326 approved on 1/12/2015.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kasfi, K., Taheri, P., Jafarpour, B. et al. Characterization of antagonistic microorganisms against Aspergillus spp. from grapevine leaf and berry surfaces. J Plant Pathol 100, 179–190 (2018). https://doi.org/10.1007/s42161-018-0042-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-018-0042-x