Abstract
Objective
Obesity and renal disease are both associated with low serum 25(OH)D. The aims of the present study were to (a) assess vitamin D status and compare serum vitamin D levels in overweight/obese versus normal-weight individuals according to eGFR and (b) assess the role of 25(OH)D in the development of secondary hyperparathyroidism (SHPT).
Design
Serum 25(OH)D, 1,25(OH)2D, parathyroid hormone (PTH), calcium, and phosphate were measured in 104 subjects with BMI > 25 kg/m2. Participants were categorized according to eGFR (ml/min/1.73m2): G1 ≥ 60 (n = 53), G2 30–59 (n = 35), and G3 15–29 (n = 16). Fifty normal-weight individuals with comparable eGFR served as controls: G1-nw (n = 23), G2-nw (n = 18), and G3-nw (n = 9).
Results
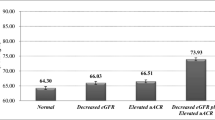
25(OH)D levels were lower in G1 compared to those in G1-nw (21.7 ± 6.5 vs 26.5 ± 7.0 ng/ml, p = 0.005), G2 versus G2-nw (19.0 ± 6.0 vs 25.0 ± 5.2 ng/ml, p = 0.001), and G3 vs G3-nw (15.8 ± 4.7 vs 20.3 ± 4.5 ng/ml, p = 0.030). 1,25(OH)2D and PTH levels were similar in obese/overweight versus normal-weight individuals in each of the eGFR categories. Factors independently associated with low 25(OH)D levels were BMI > 25 kg/m2, lower eGFR, and female gender. Mean 25(OH)D levels were < 30 ng/ml in both overweight and controls, in all eGFR groups. SHPT was universally observed when eGFR was < 30 ml/min/1.73m2.
Conclusions
Lower serum 25(OH)D but similar 1,25(OH)2D and PTH levels were observed in overweight/obese compared to normal-weight individuals. Even though vitamin D insufficiency was common across all eGFR categories, secondary hyperparathyroidism was more prevalent as eGFR declined.
Similar content being viewed by others
Introduction
Obesity has reached epidemic proportions and constitutes a major public health problem; recent data from the European region suggest that more than half of adults are either overweight (35.7%) or obese (15.9%) [1]. In addition, obesity affects more than a third of chronic kidney disease (CKD) patients and has detrimental effects on kidney structure and function [2]. In numerous epidemiological studies, obesity is consistently associated with low serum vitamin D levels [3,4,5].
The concentration of 25-hydroxyvitamin D [25(OH)D] in serum is considered as the best measure of body vitamin D status. 25(OH)D is the substrate for 1,25(OH)2D production (the active vitamin D form) following 1a hydroxylation in the renal tubules but also in extrarenal sites. 1,25(OH)2D, among its numerous other actions, increases intestinal calcium and phosphate absorption and calcium reabsorption from the glomerular filtrate. Most authorities define vitamin D insufficiency as serum 25(OH)D < 30 ng/ml [6]. By this definition, it is estimated that more than 20–50% of the general population and 70–80% of CKD patients have insufficient serum levels of vitamin D [7]. Current clinical guidelines suggest vitamin D supplementation in CKD stages 3 and 4 patients [i.e., those with estimated GFR (eGFR) of 30–59 and 15–29 ml/min/1.73 m2, respectively] using the same threshold and strategies as in the general population [8], even though this may not result in effective suppression of serum PTH [9]. Nevertheless, recent data indicate that correction of low serum 25(OH)D levels may be beneficial in individuals with CKD, leading to improvements in endothelial dysfunction [10, 11] and albuminuria [12]. Furthermore, the role of vitamin D is crucial in the pathogenesis of chronic kidney disease-mineral and bone disorder (CKD-MBD), a clinical syndrome encompassing the multiple bone, mineral, and calcific vascular abnormalities that occur during the course of CKD. As eGFR declines, low vitamin D levels may contribute to disturbances of calcium and phosphate homeostasis and development of secondary hyperparathyroidism (SHPT) [8].
Limited data are available on the vitamin D status in overweight/obese individuals and its association with eGFR [13]. The present study examined (a) serum 25(OH)D and 1,25(OH)2D concentrations in overweight/obese versus normal-weight individuals with normal to severely impaired renal function and (b) the role of 25(OH)D insufficiency in the development of secondary hyperparathyroidism in overweight/obese versus normal-weight individuals across various eGFR levels.
Subjects and methodology
Subjects
Study participants were 104 consecutive adult subjects with body mass index (BMI) ≥ 25 kg/m2, who visited the Nephrology and Hypertension Outpatient clinic of the University Hospital of Ioannina and agreed to participate in the study. The same physician was responsible for subject recruitment in the outpatient department, on a regular, 1-day-per-week basis over a 12-month period.
Obesity was defined as BMI ≥ 30 kg/m2 and overweight as BMI 25–29.9 kg/m2 [14]. Patients with a history of any major cardiovascular event in the previous 3 months (new onset angina, acute myocardial infarction, acute pulmonary edema, and cerebrovascular event), active infection, abnormal liver function, and active malignancy as well as those taking vitamin D supplements, medications affecting vitamin D metabolism, calcium supplements, or glucocorticoids were excluded from the study. Overweight/obese subjects were categorized in three groups according to their eGFR (ml/min/1.73 m2), by the abbreviated MDRD (modification of diet in renal disease) equation [15]. Group G1 ≥ 60 (n = 53), Group G2 30–59 (n = 35), and Group G3 15–29 ml/min/1.73 m2 (n = 16). A control group of 50 normal-weight subjects (BMI < 25 kg/m2) was included and participants were categorized according to eGFR: Group G1-nw ≥ 60 (n = 23), Group G2-nw 30–59 (n = 18), and Group G3-nw: 15–29 ml/min/1.73 m2 (n = 9) as well. Study subjects were categorized based solely on eGFR and not on the presence of CKD as defined in the Clinical Practice Guidelines for Chronic Kidney Disease (Kidney Disease Outcomes Quality Initiative) [16].
Patients with fasting plasma glucose of ≥ 126 mg/dl and those on antidiabetic therapy were considered as suffering from diabetes mellitus (DM). Subjects whose fasting plasma glucose was ≥ 100 mg/dl but < 126 mg/dl were considered as having impaired fasting glucose (IFG) and participants with fasting plasma glucose < 100 mg/dl as having normal fasting glucose (NFG) [17].
Mean blood pressure values were calculated from three readings obtained with the use of an electronic sphygmomanometer. Blood pressure was measured in both arms, in the sitting position, after a 10-min rest and the highest was recorded. Hypertension was defined as arterial blood pressure ≥ 140/90 mmHg or use of antihypertensive therapy. According to ATP III guidelines, low-density lipoprotein cholesterol (LDL-C) levels < 100 mg/dl were considered as optimal, and triglycerides (TG) < 150 mg/dl and high-density lipoprotein cholesterol (HDL-C) > 40 mg/dl for men and > 50 mg/dl for women were considered as normal [18].
Body weight of the study participants was measured by using the same calibrated scales and their body mass index (BMI, kg/m2) was calculated. Waist circumference was measured midway between the lower rib and iliac crest. Blood and urine samples were obtained in the morning, following an overnight 12-h fast.
Biochemical measures
Blood samples were analyzed to measure serum concentrations of creatinine (reference range 0.6–1.2 mg/dl), glucose (70–125 mg/dl), total cholesterol (TC) (110–200 mg/dl), HDL-C (35–70 mg/dl), TG (40–175 mg/dl), phosphate (2.5–5 mg/dl), calcium (8.2–10.6 mg/dl), and albumin (3.4–5 g/dl). Urine samples were analyzed for protein and creatinine and the protein to creatinine ratio was calculated (normal < 0.15 mg/mg).
Serum 25(OH)D and 1,25(OH)2D were determined quantitatively by enzyme immunoassays (IDS Systems Ltd., UK). The sensitivities of the methods for the two forms were 2 ng/ml and 2.5 pg/ml, respectively. The intra- and inter-assay CVs for 25(OH)D were 5.3 and 4.6% at the level of 16 ng/ml, and the reference range was 20–70 ng/ml. For 1,25(OH)2D, the respective CVs were 10.5 and 17% at the level of 22 pg/ml, and the reference range was 20–100 pg/ml. Intact PTH (reference range 12–72 pg/ml) was measured by a solid-phase, chemiluminescent immunometric assay with IMMULITE 1000 analyzer.
Statistical analysis
Data were expressed as mean ± standard deviation for normally distributed variables, as median (25th, 75th quartiles) for non-normally distributed variables, or as percentage frequency (for binary variables). Correlations between 25(OH)D, 1,25(OH)2D, PTH, and eGFR were assessed by using Pearson’s or Spearman’s correlation coefficient, as appropriate. For comparisons among groups, the chi square test for trend was used for quantitative variables. The independent sample t test or Mann-Whitney U test was used for comparisons between two groups of patients as appropriate.
Univariate and multivariate linear regression models were used to test the association between the assessed variables (age, gender, eGFR, BMI ≥ 25 kg/m2, serum phosphate, serum calcium, urine albumin/creatinine ratio) and the study outcomes (vitamin D and PTH). Variables with p < 0.1 in univariate analyses entered the multivariate linear regression models. In linear regression models, data were expressed as unstandardized regression coefficient (b and 95% confidence interval), standardized regression coefficient (beta), and p value. Statistical significance was set at 0.05 in all cases. Statistical analysis was performed with SPSS Version 19.0 statistical software package.
Results
Demographics and characteristics of the study subjects
The demographics and characteristics of the study population are shown in Table 1. In the overweight/obese subgroups, individuals in group G1 were younger than those in G2 and G3 (58 ± 12 versus 67 ± 11 and 68 ± 7 years, p = 0.001 and p = 0.005 respectively). No differences were observed among the three subgroups in terms of body weight, BMI, and waist circumference (p = ns). Age and somatometric characteristics were similar among those of the three normal-weight control subgroups (p = ns).
The prevalence of hypertension was higher in the overweight/obese subjects, compared to that in normal-weight controls (95 versus 80%, p = 0.003). Almost half of the overweight/obese study subjects (45%) were receiving lipid-lowering medication (statins), compared to 26% in the normal-weight group (p = 0.027). TG levels were higher and HDL lower in the overweight/obese compared to normal-weight subjects (p < 0.001). Fasting glucose level and the prevalence of diabetes or IFG were higher in the overweight/obese compared to normal-weight control subjects (104 versus 97 mg/dl, p = 0.026 and 58 versus 36%, p = 0.006, respectively).
Vitamin D and PTH
The primary goal of the study was to assess the differences in 25(OH)D levels between overweight/obese and normal-weight controls with comparable eGFR (Table 2). The mean serum 25(OH)D level was lower in overweight/obese versus that of normal-weight controls in all eGFR groups; G1 compared to G1-nw 21.7 ± 6.5 vs 26.5 ± 7.0 ng/ml, p = 0.005), G2 versus G2-nw (19.0 ± 6.0 vs 25.1 ± 5.2 ng/ml, p = 0.001), and G3 vs G3-nw (15.8 ± 4.7 vs 20.3 ± 4.5 ng/ml, p = 0.030). By contrast, mean 1,25(OH)2D level was similar in obese/overweight and normal-weight control individuals in all three eGFR groups, and the same pattern was observed with serum PTH levels (Table 2).
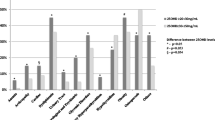
The mean level of 25(OH)D in overweight/obese subjects was 19.9 ± 6.4 ng/ml, whereas median 1,25(OH)2D was 48.4 (34.2, 60.9) pg/ml. In normal-weight subjects, mean serum 25(OH)D was 24.9 ± 6.3 ng/ml and 1,25(OH)2D was 46.3 (38.6, 61.3) pg/ml. Notably, mean 25(OH)D level in 97 out of 104 (93%) of overweight/obese individuals was suboptimal (< 30 ng/ml), whereas in normal-weight individuals, the prevalence of vitamin D insufficiency was still high (37 out of 50 participants, 74%) but lower compared to those who were overweight/obese (p < 0.001). In particular, the prevalence of vitamin D insufficiency was 89% in group G1 compared to 61% in group G1-nw (p < 0.01), and 97% in group G2 compared to 78% in group G2-nw (p = 0.02). All subjects with eGFR < 30 ml/min/1.73m2 (group G3 and G3-nw) had suboptimal 25(OH)D levels irrespective of BMI.
In the whole study population (n = 154), 25(OH)D levels measured between May and October were 6.2% higher compared to those measured between November and April (22.3 ± 6.0 versus 21.0 ± 7.2 ng/ml, respectively); this difference did not reach statistical significance (p = 0.238). A small but statistically significant difference in 25(OH)D levels was observed between females and male subjects (20.1 ± 5.9 vs 22.8 ± 7.3 ng/ml, respectively, p = 0.014). No association was found between age and serum 25(OH)D (r = − 0.117, p = 0.148).
25(OH)D levels decreased as eGFR declined (r = 0.270, p = 0.006), with G3 subjects having significantly lower levels compared to G1 (mean 15.8 ± 4.7 vs 21.7 ± 6.5 ng/ml, p = 0.004). Similar findings were observed in normal-weight individuals; G1-nw participants had higher 25(OH)D levels compared to G3-nw participants (mean 26.5 ± 7.0 vs 20.3 ± 4.5 ng/ml, p = 0.039). Multivariate regression analysis in all study participants showed a significant negative effect of the presence of overweight/obesity (BMI > 25 kg/m2) (p < 0.001), female gender (p = 0.023), raised serum triglycerides (p < 0.001), and low eGFR on 25(OH)D levels (p = 0.001) (Table 3).
In the overweight/obese individuals, the association between 1,25(OH)2D and eGFR was even stronger (r = 0.412, p < 0.001) than that of 25(OH)D, and this was also the case for normal-weight controls (r = 0.490, p < 0.001). G1 subjects had higher median 1,25(OH)2D levels compared to G2 and G3 patients [58.0 (44.3, 67.9) vs 43.9 (31.5, 56.0) and 28.3 (23.3, 44.1) pg/ml, p = 0.002 and p < 0.001, respectively]. Similarly, G1-nw subjects had higher 1,25(OH)2D levels compared to G2-nw and G3-nw subjects [59.2 (43.8, 78.2) vs 45.3 (38.7, 49.6) and 39.1 (33.2, 39.3) pg/ml, p = 0.022 and p = 0.001, respectively]. Multivariate regression analysis confirmed this finding with eGFR showing a strong positive influence on 1,25(OH)2D levels (p < 0.001). Significant correlations with serum phosphate (p = 0.004) and 25(OH)D (p = 0.005) were also found (Table 4).
Serum PTH levels rose progressively as eGFR declined in both overweight/obese and normal-weight subjects (r = − 0.531, p < 0.001 and r = − 0.661, p < 0.001, respectively). SHPT began to occur in eGFR levels < 60 ml/min/1.73m2; almost half of the participants with eGFR 30–59 ml/min/1.73m2 (16 out of 35) had PTH levels above the upper limit of the normal reference range. Notably, most of them (14/16 or 87.5%) had eGFR < 45 ml/min/1.73m2. All patients with eGFR < 30 ml/min/1.73m2 in the obese/overweight group had PTH values above the reference range. Patients in group G3 had higher median PTH levels [161.0 (104.0, 345.6)] compared to G1 [54.0 (44.4, 78.1), p < 0.001] and G2 patients [61.1 (48.1, 111.0), p < 0.001] and a similar pattern was observed in the normal-weight group; G3-nw had higher PTH [182.0 (164.0, 267.0) pg/ml] compared to G1-nw [48.3 (35.4, 74.2), p < 0.001] and G2-nw [80.5 (38.3, 106.0), p < 0.001]. In multivariate analysis, eGFR < 30 ml/min/1.73m2 (p < 0.001), serum phosphate (p = 0.039), and serum calcium (p = 0.040) were significantly associated with PTH (Table 5).
Serum calcium levels were similar in the three overweight/obese subgroups (Table 1). In normal-weight subjects, lower calcium levels were observed in the G3-nw (8.8 ± 0.7 mg/dl) compared to G2-nw (9.4 ± 0.5 mg/dl, p = 0.007) and G1-nw (9.5 ± 0.4, p = 0.001) groups. Serum phosphate was higher in G3 (3.7 ± 0.5 mg/dl) compared to that in G2 (3.2 ± 0.6 mg/dl, p = 0.017) and G1 (3.2 ± 0.5, p = 0.019) and the same pattern was observed in normal-weight controls [4.2 ± 0.6 mg/dl in G3-nw compared to 3.3 ± 0.7 mg/dl in G2-nw (p = 0.005) and 3.0 ± 0.6 in G1-nw (p < 0.001)].
Overweight/obese subjects with DM had lower 25(OH)D compared to those with normal fasting glucose (NFG) (17.8 ± 5.6 vs 21.0 ± 6.5 ng/ml, p = 0.035), lower 1,25(OH)2D [40.9 (31.4, 56.0) vs 53.8 (43.3, 67.9) pg/ml, p = 0.031], and higher PTH values [82.0 (55.3, 127.0) vs 55.79 (48.0, 97.0) pg/ml, p = 0.041]. Of note, subjects with diabetes were older and had a higher mean BMI compared to those with NFG, but no statistically significant differences were observed between the two groups in terms of mean eGFR (Table 6). Overweight/obese subjects with IFG had lower 1,25(OH)2D levels compared to subjects with normal fasting glucose (p < 0.042). No differences in 25(OH)D, 1,25(OH)2D, or PTH levels were observed between normal-weight subjects with or without DM.
Discussion
The findings of this study suggest that serum levels of 25(OH)D, the primary indicator of vitamin D body stores, are considerably lower in obese/overweight subjects in comparison to those of normal-weight controls. Despite the observed lower 25(OH)D levels, 1,25(OH)2D and PTH levels were similar compared to those of normal-weight controls, in each eGFR category. Both 25(OH)D and 1,25(OH)2D levels progressively decreased as eGFR deteriorated in overweight/obese as well as in normal-weight subjects. Serum PTH was inversely associated with eGFR, but even though mean 25(OH)D levels were lower than currently recommended in all overweight/obese and normal-weight eGFR subgroups, median PTH exceeded the upper limit of normal only in the groups with eGFR of < 60 ml/min/1.73m2.
The mean 25(OH)D level was 19.9 ng/ml in the overweight/obese subjects and suboptimal vitamin D levels (< 30 ng/ml) were present in almost all (93%) overweight/obese and in the majority (74%) of the control subjects. Previous studies in Greek populations have found rates of vitamin D deficiency (levels < 20 ng/ml) as high as 96% among community-living elderly in Athens [19], whereas mean values of 16.8 ng/ml (42 nmol/l) were reported in obese women (mean age 40.6 years) [20]. The findings of a recent large cross-sectional study from Crete were consistent with our observations; results from 8042 serum 25(OH)D measurements over a 5-year period revealed suboptimal 25(OH)D values in both males (19.48 ± 9.51 ng/ml) and females (18.01 ± 9.01 ng/ml), and lower levels were observed in patients with a biochemical profile indicative of metabolic syndrome [21].
Although the exact mechanism responsible for the low serum calcidiol levels in obesity remains unclear, a number of factors have been implicated, such as sequestration of vitamin D in adipose tissue, limited body surface area exposed to sunlight due to dressing habits, decreased outdoor activity, and lower calcium and vitamin D intake in obese individuals. It has been postulated that the larger whole body adipose tissue volume of distribution may be responsible for the lower serum 25(OH)D [22, 23]. Lack of accurate methods capable of measuring and separating active from inactive forms of vitamin D may be another possible explanation. New assays capable of measuring different forms of vitamin D along with vitamin D epimers or new biomarkers may estimate vitamin D status more accurately [24].
By categorizing the overweight/obese and normal-weight study subjects according to eGFR, we were able to determine the changes of vitamin D concentrations across eGFR levels. In both overweight/obese and in normal-weight subjects, lower 25(OH)D and 1,25(OH)2D levels were observed in subjects with low (< 30 ml/min/1.73m2) compared to those with higher eGFR. These results are in agreement with previous observations from studies assessing vitamin D status in CKD populations. Low 25(OH)D levels are prevalent in CKD, although there is a lack of consensus on the definition of vitamin D deficiency and insufficiency, as well as significant variability, depending on the studied population; a mean 25(OH)D level of 28.6 ng/ml was observed in Brasil [13], whereas the mean level in a Southeast Asian population was 20.1 ng/ml [25]. Most observational studies report a significant reduction in 25(OH)D levels when eGFR falls below 30 ml/min/1.73 m2.26 with a reported 61% of patients having 25(OH)D < 20 ml/min/1.73m2 [27]. In our study population, when eGFR was < 30 ml/min/1.73 m2, mean vitamin D levels of 15.8 and 20.3 ng/ml were observed in the overweight/obese and normal-weight subjects, respectively. Another study by Guessous et al. confirmed the high prevalence of suboptimal (< 30 ng/ml) levels of 25(OH)D in 135 patients with CKD, although interestingly, the mean 25(OH)D level and prevalence of 25(OH)D insufficiency were comparable to those of 1,010 participants without CKD [28]. Only one of the abovementioned studies focused on obese/overweight subjects [13]. To our knowledge, the present study is the first to examine 25(OH)D and 1,25(OH)2 levels and their impact on serum PTH in both obese and normal-weight individuals with normal to low eGFR.
Factors contributing to the low serum 25(OH)D in CKD include the hyperpigmentation of patients with advanced kidney disease resulting in impaired dermal synthesis of endogenous vitamin D and the dietary restrictions resulting in decreased vitamin D intake. Furthermore, heavy proteinuria may be accompanied by high urinary loss of vitamin D-binding protein and renal loss of vitamin D [7].
Even though mean 25(OH)D levels were suboptimal in all obese/overweight and normal-weight eGFR subgroups, median serum PTH was within the normal range in those with eGFR > 60 ml/min/1.73m2. The fact that the observed differences in 25(OH)D were not accompanied by higher PTH raises the possibility that the observed suboptimal serum 25(OH)D levels may not reflect true vitamin D insufficiency in our study population. Moreover, the currently accepted threshold for vitamin D adequacy (> 30 ng/ml) remains controversial; indeed, in a large cross-sectional study in more than 8,000 subjects living on the island of Crete in Greece, normalization of PTH levels was observed at 25(OH)D levels of ~ 20 ng/ml [21]. Furthermore, a report from the Institute of Medicine committee concluded that in terms of bone health, most individuals meet their needs for vitamin D at 25(OH)D levels of 20 ng/ml [29].
The lack of association between 25(OH)D levels and PTH has been noted in several studies in CKD populations. In a large study of 1,814 CKD patients, Levin et al. found that the prevalence of 25(OH)D deficiency remained relatively stable until eGFR fell below 30 ml/min/1.73m2 and appeared to be dissociated from the prevalence of SHPT [26]. In addition, the observed interaction between 25(OH)D and 1,25(OH)2D disappeared when eGFR was < 60 ml/min/1.73m2, a finding that has been reported in other CKD studies [30]. Moreover, Silva et al. did not find any differences in median PTH in 244 obese/overweight patients with mean eGFR = 29 ml/min/1.73m2 among participants with sufficient (> 30 ng/ml), insufficient (20–30 ng/ml), and deficient (< 20 ng/ml) 25(OH)D levels. In our study, almost half of the participants with eGFR 30–59 ml/min/1.73m2 (16 out of 35) had PTH levels above the upper limit of the normal reference range (12–72 pg/ml), and most of them (14/16 or 87.5%) had eGFR < 45 ml/min/1.73m2, consistent with the findings of the study by Levin et al., where SHPT also occurred at eGFR levels of less than 45 ml/min/1.73m2 [26]. In addition, our data suggest that SHPT was more prevalent in those with eGFR < 30 ml/min/1.73m2, when PTH levels were above the upper limit of the reference range in all subjects. In advanced stage 4 CKD, a number of other relevant abnormalities exist—apart from the low 25(OH)D levels—that could potentially contribute to hyperparathyroidism; indeed, in patients with eGFR < 30 ml/min/1.73m2 in our study, levels of 1,25(OH)2D were also reduced and mean serum phosphate was higher (but not exceeding the normal range). In addition, advanced CKD is associated with increased fibroblast growth factor-23 (FGF-23) levels, downregulation of tissue vitamin D receptors, and skeletal resistance to the calcemic actions of PTH (not assessed in the present study).
The 2003 Kidney Disease Outcomes Quality Initiative (KDOQI) as well as the 2017 Kidney Disease/Improving Global Outcomes (KDIGO) guidelines recommended routine screening of 25(OH)D levels for patients with CKD and SHPT and advocated the correction of vitamin D insufficiency or deficiency [7, 8]. There is however limited evidence that supplementation to correct calcidiol levels will correct the SHPT in CKD patients [31]. In a study of vitamin D-deficient CKD patients treated with high doses of ergocalciferol (50,000 IU once weekly for 12 weeks followed by 50,000 IU per month for a total of 6 months), only about 50% of patients achieved adequate levels of 25(OH)D [32]. When again high doses of ergocalciferol were administered to 52 CKD patients to normalize 25(OH)D levels, stage 3 CKD patients (GFR 30–59 ml/min/1.73m2) showed a significant improvement in 1,25(OH)2D and a reduction in PTH levels, but no significant effect was observed in stage 4 CKD patients (GFR 15–29 ml/min/1.73m2) [33]. It is therefore conceivable that at earlier stages of CKD, the production of 1,25(OH)2D is reduced due to the low levels of 25(OH)D, but as kidney disease progresses, other factors such as the reduction of renal mass, reduced 1-alpha-hydroxylase action, and elevated FGF-23 are also involved.
Our observation of lower serum 25(OH)D and higher PTH levels in overweight/obese subjects with type 2 diabetes compared to those with normal fasting glucose is consistent with a large body of literature [34]. The observed differences could be partly explained by the higher BMI of patients with diabetes compared to those with NFG (32.6 ± 4.5 vs 29.4 ± 2.8 kg/m2, p = 0.002) Our finding of lower serum 1,25(OH)2D and higher PTH values in subjects with diabetes compared to those with normal fasting glucose is of interest; there is evidence that 1,25(OH)2D regulates insulin secretion from pancreatic β cells by inducing calcium oscillations and insulin release [35]. On the other hand, the fact that no differences in serum 25(OH)D, 1,25(OH)2D, or PTH levels were observed between normal-weight subjects with or without DM would imply that the primary regulating factor is the presence of obesity, rather than diabetes per se.
Finally, female gender was associated with lower 25(OH)D levels. This small but statistically significant difference is consistent with previous findings [21]. A possible contributing factor could be the fact that females had higher BMI (29.5 ± 5.3 kg/m2 vs 27.6 ± 3.2 kg/m2, p = 0.009). Age did not seem to affect 25(OH)D levels in the present study, possibly as a result of the low number of participants of very young (children and adolescents) or very old age (> 70 years).
Limitations of the present study include the relatively small number of subjects and the lack of FGF-23 or vitamin D-binding protein measurement. In addition, the younger age of subjects in group G1 compared to those with lower GFR, is a potential source of bias. As sample collection for 25(OH)D measurements was carried out throughout the year, seasonal variation of serum 25(OH)D levels could have been another potential source of bias, even though no significant differences were observed in our sample population. The absence of seasonal variation in obese patients has been reported by others [36] and could be attributed to sequestration of vitamin D in fat tissue, masking the seasonal variation in obesity [21]. Notably, the region of Epirus where the study was carried out has the biggest annual rainfall in Greece; therefore, inadequate sun exposure may have been an additional factor that may explain the observed significant proportion of subjects with low serum vitamin D levels.
Conclusions
In summary, we found that serum 25(OH)D levels, the most representative marker of vitamin D body stores, are lower in obese/overweight compared to normal-weight subjects, irrespective of eGFR. Even though vitamin D insufficiency was prominent in all eGFR subgroups, secondary hyperparathyroidism develops only when eGFR falls below 60 ml/min and is universally observed in those with eGFR < 30 ml/min/1.73m2, when, in addition to low 25(OH)D, 1,25(OH)2D levels are also reduced and serum P levels increase.
References
European health interview survey. http://ec.europa.eu/eurostat. Published online October 20, 2016
Eknoyan G (2011) Obesity and chronic kidney disease. Nefrologia 31(4):397–403
Vanlint S (2013) Vitamin D and obesity. Nutrients Mar 5(3):949–956
Parikh SJ, Edelman M, Uwaifo GI et al (2004) The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab 89(3):1196–1199
Lagunova Z, Porojnicu A, Lindberg F, Hexeberg S, Moan J (2009) The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res 29(9):3713–3720
Holick MF, Binkley NC, Bischoff-Ferrari HA et al (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96(7):1911–1930
Nigwekar SU, Bhan I, Thadhani R (2012) Ergocalciferol and cholecalciferol in CKD. Am J Kidney Dis 60(1):139–156
KDIGO (2017) Clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl 7:S1–S59
Kramer H, Berns JS, Choi MJ, Martin K, Rocco MV (2014) 25-Hydroxyvitamin D testing and supplementation in CKD: an NKF-KDOQI controversies report. Am J Kidney Dis 64(4):499–509
Lundwall K, Jorneskog G, Jacobson SH, Spaak J (2015) Paricalcitol, microvascular and endothelial function in non-diabetic chronic kidney disease: a randomized trial. Am J Nephrol 42(4):265–273
Zoccali C, Curatola G, Panuccio V et al (2014) Paricalcitol and endothelial function in chronic kidney disease trial. Hypertension 64(5):1005–1011
Molina P, Gorriz JL, Molina MD et al (2014) The effect of cholecalciferol for lowering albuminuria in chronic kidney disease: a prospective controlled study. Nephrol Dial Transplant 29(1):97–109
Barreto Silva MI, Cavalieri VV, Lemos CC, Klein MR, Bregman R (2017) Body adiposity predictors of vitamin D status in nondialyzed patients with chronic kidney disease: a cross-sectional analysis in a tropical climate city. Nutrition 33:240–247
World Health Organization 2000 Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. WHO Technical Report Series (894). Geneva, Switzerland: WHO. Available at: http://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 16 130(6):461–470
National Kidney Foundation (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39(2 Suppl 1):S1
American Diabetes Association (2010) Diagnosis and classification of diabetes mellitus. Diabetes Care 33(Suppl 1):S62–S69
Third Report of the National Cholesterol Education Program (NCEP) (2002) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 17, 106(25):3143–3421
Papapetrou PD, Triantafyllopoulou M, Korakovouni A (2008) Severe vitamin D deficiency in the institutionalized elderly. J Endocrinol Investig 31(9):784–787
Tzotzas T, Papadopoulou FG, Tziomalos K et al (2010) Rising serum 25-hydroxy-vitamin D levels after weight loss in obese women correlate with improvement in insulin resistance. J Clin Endocrinol Metab 95(9):4251–4257
Katrinaki M, Kampa M, Margioris A, Castanas E, Malliaraki N (2016) Vitamin D levels in a large Mediterranean cohort: reconsidering normal cut-off values. Hormones (Athens) 15(2):205–223
Guasch A, Bulló M, Rabassa A, Bonada A, Del Castillo D, Sabench F, Salas-Salvadó J (2012) Plasma vitamin D and parathormone are associated with obesity and atherogenic dyslipidemia: a cross-sectional study. Cardiovasc Diabetol 11(11):149
Walsh JS, Evans AL, Bowles S et al (2016) Free 25-hydroxyvitamin D is low in obesity, but there are no adverse associations with bone health. Am J Clin Nutr 103(6):1465–1471
Shah I, Petroczi A, Naughton DP (2012) Method for simultaneous analysis of eight analogues of vitamin D using liquid chromatography tandem mass spectrometry. Chem Cent J 1;6(1):112
Ngai M, Lin V, Wong HC, Vathsala A, How P (2014) Vitamin D status and its association with mineral and bone disorder in a multi-ethnic chronic kidney disease population. Clin Nephrol 82(4):231–239
Levin A, Bakris GL, Molitch M et al (2007) Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphate in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 71(1):31–38
Kim SM, Choi HJ, Lee JP et al (2014) Prevalence of vitamin D deficiency and effects of supplementation with cholecalciferol in patients with chronic kidney disease. J Ren Nutr 24(1):20–25
Guessous I, McClellan W, Kleinbaum D et al (2014) Comparisons of serum vitamin D levels, status, and determinants in populations with and without chronic kidney disease not requiring renal dialysis: a 24-hour urine collection population-based study. J Ren Nutr 24(5):303–312
Ross AC, Manson JE, Abrams SA et al (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96(1):53–58
Ishimura E, Nishizawa Y, Inaba M et al (1999) Serum levels of 1,25- dihydroxyvitamin D, 24,25-dihydroxyvitamin D, and 25-hydroxyvitamin D in nondialyzed patients with chronic renal failure. Kidney Int 55:1019–1027
Martin KJ, González EA (2011) Vitamin D supplementation in CKD. Clin Nephrol 75(4):286–293
Al-Aly Z, Qazi RA, González EA, Zeringue A, Martin KJ (2007) Changes in serum 25-hydroxyvitamin D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKD. Am J Kidney Dis 50(1):59–68
Zisman AL, Hristova M, Ho LT, Sprague SM (2007) Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol 27(1):36–43
Afzal S, Bojesen SE, Nordestgaard BG (2013) Low 25-hydroxyvitamin D and risk of type 2 diabetes: a prospective cohort study and metaanalysis. Clin Chem 59(2):381–391
Sergeev IN (2016) 1,25-Dihydroxyvitamin D3 and type 2 diabetes: Ca2+−dependent molecular mechanisms and the role of vitamin D status. Horm Mol Biol Clin Investig 26(1):61–65
Bolland MJ, Grey AB, Ames RW (2007) The effects of seasonal variation of 25-hydroxyvitamin D and fat mass on a diagnosis of vitamin D sufficiency. Am J Clin Nutr 86(4):959–6428
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee of Ioannina University Hospital.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Submission declaration and verification
This work has not been published previously and it is not under consideration for publication elsewhere. The publication is approved by all authors and, if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically, without the written consent of the copyright-holder.
Rights and permissions
About this article
Cite this article
Kitsos, A., Dounousi, E., Kalaitzidis, R. et al. Serum vitamin D in obese and overweight subjects according to estimated glomerular filtration rate. Hormones 17, 237–246 (2018). https://doi.org/10.1007/s42000-018-0022-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42000-018-0022-8