Abstract
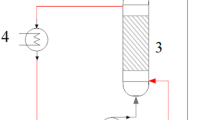
Candida antarctica lipase B (CALB) was cross-linked efficiently, using a reagent containing glutaraldehyde (GA), paraformaldehyde (PA), and a polylysine reagent. For the reaction under micro-flow conditions, CALB was immobilized on the inner surface of a polytetrafluoroethylene (PTFE) tube, by flowing the cross-linking reagents in an appropriate flow rate. The immobilized CALB formed a membrane-like structure on the inner surface of the tube and showed catalytic activity for transesterification. Although the enzyme activity was not high at the immobilized CALB, the productivity of micro-flow reaction using immobilized CALB was higher than that of batch-wise reaction using free CALB.

Graphical Abstract



Similar content being viewed by others
References
Polaina J, MacCabe AP (2010) Industrial enzymes. Springer, Dordrecht
Casas-Godoy L, Meunchan M, Cot M, Duquesne S, Bordes F, Marty A (2014) J Biotechnol 180:30–36
Chang SW, Shaw JF, Yang KH, Shih IL, Hsieh CH, Shieh CJ (2005) Green Chem 7:547–551
Gutmann B, Cantillo D, Kappe O (2015) Angew Chem Int Ed 54:6688–6728
Heintz S, Mitic A, Ringborg RH, Krühne U, Woodley JM, Gernaey KV (2016) J Flow Chem 6:18–26
Jime’nez-Gonza’lez C, Poechlauer P, Broxterman QB, Yang BS, Ende D, Baird J, Bertsch C, Hannah RE, Dell’Orco P, Manley J (2011) Org Process Res Dev 15:900–911
Novak U, Lavric D, Žnidaršič-Plazl P (2016) J Flow Chem 6:33–38
Wohlgemuth R, Plazl I, Žnidaršič-Plazl P, Gernaey KV, Woodley JM (2015) Trends Biotechnol 33:302–314
Žnidaršič-Plazl P (2017) J Flow Chem 7:111–117
Fu H, Dencic I, Tibhe J, Sanchez Pedraza CA, Wang Q, Noël T, Meuldijk J, Croon M, Hessel V, Diels L (2012) Chem Eng J 207:564–576
Denčić I, Noël T, Meuldijk J, Croon M, Hessel V (2013) Eng Life Sci 13:326–343
Honda T, Miyazaki M, Nakamura H, Maeda H (2005) Chem Commun:5062–5064
Honda T, Miyazaki M, Nakamura H, Maeda H (2006) Adv Synth Catal 348:2163–2171
Doukyu N, Ogino H (2010) Biochem Eng J 48:270–282
Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente R (2007) Enzym Microb Technol 40:1451–1463
Sheldon RA (2007) Adv Synth Catal 349:1289–1307
Yoshimura Y, Osaki S, Miyake Y, Mori H (2013) Reports of Industrial Technology Center of Wakayama Prefecture, pp 11–12
Yoshimura Y, Tsuchitani A, Mori M, Osaki S, Miyazaki T, Mori H (2016) The 96th CSJ Annual Meeting, 3PA-231
Bolivar JM, Eisl I, Nidetzky B (2015) Catal Today 259:66–80
Wold F (1972) Methods Enzymol 25:623–651
Karnovsky MJ (1965) J Cell Biol 27:137A–138A
Barbosa O, Torres R, Ortiz C, Fernandez-Lafuentec R (2012) Process Biochem 47:766–774
Uppenberg J, Hansen MT, Patkar S, Jones TA (1994) Structure 2:293–308
Ishii M (1989) WO Patent 1988002775 A1
Alotaibi M, Manayil JC, Greenway GM, Haswell SJ, Kelly SM, Lee AF, Wilson K, Kyriakou G (2018) React Chem Eng. Advance Article
Nie K, Xie F, Wang F, Tan T (2006) J Mol Catal B Enzym 43:142–147
Bai YX, Li Y, Feng YY, Yi LX (2006) Process Biochem 41:770–777
Chen B, Hu J, Miller EM, Xie W, Cai M, Gross RA (2008) Biomacromolecules 9:463–471
Chulalaksananukul W, Condoret JS, Combes D (1992) Enzym Microb Technol 14:293–298
Yadav GD, Trivedi AH (2003) Enzym Microb Technol 32:783–789
Henderson JW, Ricker RD, Bidlingmeyer BA, Woodward C (2000) Agilent Technologies, Inc., 5980-1193E
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yoshimura, Y., Saito, A., Mori, M. et al. Immobilization of lipase on the surface of a micro tube and continuous transesterification. J Flow Chem 8, 45–50 (2018). https://doi.org/10.1007/s41981-018-0006-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41981-018-0006-5