Abstract
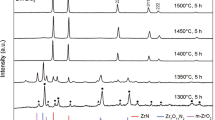
In this research, the thermodynamic aspect of the nano-sized zirconium carbide production is investigated via a facile, low-temperature and cost-effective carbothermal method under vacuum and argon atmospheres. The starting materials were zirconium acetate and sucrose as zirconium and carbon precursors, respectively. The gels were prepared based on 3, 4, 5, and 7 molar ratios of carbon to zirconium and heated at 1200 and 1400 °C under vacuum and argon atmospheres. The formation of zirconium carbides under different atmospheres were studied via thermogravimetric analysis and the results were compared. The phase composition and microstructural features were investigated using X-ray diffraction and scanning electron microscopy, respectively. According to the thermogravimetric results and performed thermodynamic calculations, it was revealed that the ZrC formation starts at 1200 °C under vacuum. It is also demonstrated that the formation of nano ZrC powder with crystallite sizes smaller than 30 nm, completely occurs after processing at 1400 °C in vacuum. The measured lattice parameter value of the optimized sample was equal to 4.7003 Å.










Similar content being viewed by others
References
Ushakov, S.V., Navrotsky, A., Hong, Q., Walle, A.: Carbides and nitrides of zirconium and hafnium. Materials. 12, 2728 (2019)
Arianpour, F., Golestanifard, F., Rezaie, H.R., Mazaheri, M., Celik, A., Kara, F., Fantozzi, G.: Processing, phase evaluation and mechanical properties of MoSi2 doped 4TaC-HfC based UHTCs consolidated by spark plasma sintering. Int. J. Refract. Met. Hard Mater. 56, 1–7 (2016)
Zhou, Y., Heitmann, T.W., Fahrenholtz, W.G., Hilmas, G.E.: Synthesis of ZrCx with controlled carbon stoichiometry by low temperature solid state reaction. J. Eur. Ceram. Soc. 39, 2594–2600 (2019)
Kim, J.H., Seo, M.: Influence of lattice strain on grain growth behavior of zirconium carbide. Ceram. Int. 44, 17204–17208 (2018)
Giorgi, E., Grasso, S., Zapata-Solvas, E., Lee, W.E.: Reactive carbothermal reduction of ZrC and ZrOC using spark plasma sintering. Adv. Appl. Ceram. S1, S34–S47 (2018)
Yu, L., Feng, L., Lee, H.I., Silvestroni, L., Sciti, D., Woo, Y.J., Lee, S.H.: Synthesis and densification of ultra-fine ZrC powders-effects of C/Zr ratio. Int. J. Refract. Met. Hard Mater. 81, 149–154 (2019)
Zhang, M.X., Hu, Q.D., Huang, B., Li, J.Z., Li, J.G.: Study of formation behavior of ZrC in the Fe-Zr-C system during combustion synthesis. Int. J. Refract. Met. Hard Mater. 29, 596–600 (2011)
Zhang, M.X., Huanga, B., Hua, Q.D., Lia, J.G.: Study of formation behavior of ZrC in the Cu-Zr-C system during combustion synthesis. Int. J. Refract. Met. Hard Mater. 31, 230–235 (2012)
Li, J., Fu, Z.Y., Wang, W.M., Wang, H., Lee, S.H., Niihara, K.: Preparation of ZrC by self-propagating high-temperature synthesis. Ceram. Int. 36, 1681–1686 (2010)
Hu, Q., Zhang, M., Luo, P., Song, M., Li, J.: Thermal explosion synthesis of ZrC particles and their mechanism of formation from Al-Zr-C elemental powders. Int. J. Refract. Met. Hard Mater. 35, 251–256 (2012)
Nam, Y.S., Cui, X.M., Jeong, L., Lee, J.Y., Park, W.H.: Fabrication and characterization of zirconium carbide (ZrC) nanofibers with thermal storage property. Thin Solid Films. 517, 6531–6538 (2009)
Combemale, L., Leconte, Y., Portier, X., Herlin-Boime, N., Reynaud, C.: Synthesis of nanosized zirconium carbide by laser pyrolysis route. J. Alloy Compound. 483, 468–472 (2009)
Dolle, M., Gosset, D., Bogicevic, C., Karolak, F., Simeone, D., Baldinozzi, G.: Synthesis of nanosized zirconium carbide by a sol-gel route. J. Eur. Ceram. Soc. 27, 2061–2067 (2007)
Zheng, Y., Zheng, Y., Wang, R., Wei, K.: Direct determination of carbothermal reduction temperature for preparing silicon carbide from the vacuum furnace thermobarogram. Vacuum. 82, 336–339 (2008)
Wu, K., Zhang, G., Gou, H., Chou, K.: Preparation and purification of titanium carbide via vacuum carbothermic reduction of ilmenite. Vacuum. 151, 51–60 (2018)
Sen, W., Sun, H., Yang, B., Xu, B., Ma, W., Liu, D., Dai, Y.: Preparation of titanium carbide powders by carbothermal reduction of titania/charcoal at vacuum condition. Int. J. Refract. Met. Hard Mater. 28, 628–632 (2010)
Arianpour, F., Kazemi, F., Rezaie, H.R., Asjodi, A., Liu, J.: Nano zirconium carbide powder synthesis via carbothermal route. Defect Diffus Forum 334, 381–386 (2013)
Sevastyanov, V.G., Simonenko, E.P., Ignatov, N.A., Ezhov, Y.S., Simonenko, N.P., Kuznetsov, N.T.: Synthesis of highly dispersed super-refractory tantalum-zirconium carbide Ta4ZrC5 and tantalum-hafnium carbide Ta4HfC5 via sol-gel technology. Russ. J. Inorg. Chem. 56, 1681–1687 (2011)
Jenkins, R., Snyder, R.: Introduction to X-ray powder diffractometery. 2nd edition. John Wiley & Sons, USA (2012)
Saberi, A., Alinejad, B., Negahdari, Z., Kazemi, F., Almasi, A.: A novel method to low temperature synthesis of nanocrystalline forsterite. Mater. Res. Bull. 42, 666–673 (2007)
Ebrahimi-Kahrizsangi, R., Amini-Kahrizsangi, E.: Zirconia carbothermal reduction: Non-isothermal kinetics. Int. J. Refract. Met. Hard Mater. 27, 637–641 (2009)
Berger, L.M., Gruner, W., Langholf, E., Stolle, S.: On the mechanism of carbothermal reduction processes of TiO2 and ZrO2. Int. J. Refract. Met. Hard Mater. 17, 235–243 (1999)
David, J., Trolliard, G., Gendre, M., Maitre, A.: TEM study of the reaction mechanisms involved in the carbothermal reduction of zirconia. J. Eur. Ceram. Soc. 33, 165–179 (2013)
Ang, C., Williams, T., Seeber, A., Wang, H., Cheng, Y.: Synthesis and evolution of zirconium carbide via sol-gel route: features of nanoparticle oxide-carbon reactions. J. Am. Ceram. Soc. 96, 1099–1106 (2013)
Shatynski, S.R.: The thermochemistry of transition metal carbides. Oxid. Met. 13, 105–118 (1979)
Guillermet, A.F.: Analysis of thermochemical properties and phase stability in the zirconium-carbon system. J. Alloys Compound. 217, 69–89 (1995)
Chase, M.W., Curnut, J.L., Downey, J.R., McDonald, R.A., Syverud, A.N., Valenzuela, E.A.: JANAF thermochemical Tables. J. Phys. Chem. Ref. Data. 11, 695–940 (1982)
Gaskell, D.R., Laughlin, D.E.: Introduction to the thermodynamics of materials. 6th ed. CRC Press, New York (2017)
Fabris, S., Paxton, A.T., Finnis, M.W.: A stabilization mechanism of zirconia based oxygen vacancies only. Acta Mater. 50, 5171–5178 (2002)
Schönfeld, K., Martin, H.P., Michaelis, A.: Pressureless sintering of ZrC with variable stoichiometry. J Adv Ceram 6, 165–175 (2017)
Chu, A., Qin, M., Rafi-ud-din, Zhang, L., Lu, H., Jia, B., Qu, X.: Carbothermal synthesis of ZrC powders using a combustion synthesis precursor. Int. J. Refract. Met. Hard Mater. 36, 204–210 (2013)
Sacks, M.D., Wang, C., Yang, Z., Jian, A.: Carbothermal reduction synthesis of nanocrystalline zirconium carbide and hafnium carbide powders using solution-derived precursors. J. Mater. Sci. 39, 6057–6066 (2004)
Kelly, J.R., Denry, I.: Stabilized zirconia as a structural ceramic: an overview. Dent Mater 4, 289–298 (2008)
Gendre, M., Maitre, A., Trolliard, G.: Synthesis of zirconium oxycarbide (ZrCxOy) powders: influence of stoichiometry on densification kinetics during spark plasma sintering and on mechanical properties. J. Eur. Ceram. Soc. 31, 2377–2385 (2011)
Feng, L., Lee, S., Lee, H.: Nano-sized zirconium carbide powder: synthesis and densification using a spark plasma sintering apparatus. Int. J. Refract. Met. Hard Mater. 64, 98–105 (2017)
Rejasse, F., Rapaud, O., Trolliard, G., Masson, O., Maitre, A.: Experimental investigation and thermodynamic evaluation of the C-O-Zr ternary system. RSC Adv. 106, 1–30 (2016)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arianpour, F., Kazemi, F. & Rezaie, H.R. Thermodynamic study of zirconium carbide synthesis via a low-temperature pyrovacuum method. J Aust Ceram Soc 56, 969–977 (2020). https://doi.org/10.1007/s41779-019-00428-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-019-00428-1