Abstract
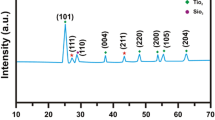
The effect of different sintering temperatures on structural and optical properties of ZnO-Zn2SiO4 composite was investigated. In this study, a ZnO-Zn2SiO4 composite was prepared by encapsulated zinc nitrate hexahydrate with amorphous silica nanoparticles in deionized water. The amorphous silica nanoparticles were prepared by reacting sodium silicate with ethanol. Zinc nitrate was mixed with the obtained amorphous silica nanoparticles with the ratio of 2:1 and 1.25:1 for Zn:Si and subjected to the different sintering temperatures from 600 to 1000 °C. Field emission scanning electron microscope (FESEM) microstructure showed that samples exhibit spherical morphology up to 700 °C and dumbbell morphology above 800 °C sintering temperature. The formation of Zn2SiO4 crystal phase appears from 700 °C and onwards together with ZnO crystal phase. The gaining of sintering temperature has also raised the amount of Zn2SiO4 phase and band gap values of the ZnO-Zn2SiO4 composite.







Similar content being viewed by others
References
Ye, S., Xiao, F., Pan, Y.X., Ma, Y.Y., Zhang, Q.Y.: Phosphors in phosphor-converted white light-emitting diodes: recent advances in materials, techniques and properties. Mater Sci Eng R. 70, 1–34 (2010)
Yen, W.M., Shionoya, S., Yamamoto, H.: Phosphors Handbooks. CRC Press, New York (2007)
Takesue, M., Hayashi, H., Smith, R.L.: Thermal and chemical methods for producing zinc silicate (willemite): a review. Prog Cryst Growth Charact Mater. 55, 98–124 (2009)
Baccile, N., Babonneau, F., Thomas, B., Coradin, T.: Introducing ecodesign in silica sol-gel materials. J Mater Chem. 19(45), 8537–8559 (2009)
Elimelech, H., Avnir, D.: Sodium-silicate route to submicrometer hybrid PEG@silica particles. Chem Mater. 20, 2224–2227 (2008)
Wu, W., Cao, S., Yuan, X., Zhao, Z., Fang, L.: Sodium silicate route: fabricating high monodisperse hollow silica spheres by a facile method. J Porous Mater. 19, 913–919 (2012)
Sundblom, A., Oliveira, C.L.P., Pedersen, J.S., Palmqvist, A.E.C.: Decoupling particle formation from intraparticle ordering in mesostructured silica colloids. Microporous Mesoporous Mater. 145(1–3), 59–64 (2011)
Yun-yu, Z., Xiao-xuan, L., Zheng-xing, C.: Rapid synthesis of well-ordered mesoporous silica from sodium silicate. Powder Technol. 226, 239–245 (2012)
Cheng, G., Liu, C.: Preparation of lamellar mesoporous silica microspheres via SDS templates. Mater Chem Phys. 77(2), 359–364 (2003)
Buining, P., Liz-Marzan, L.M., Philipse, A.P.: A simple preparation of small, smooth silica spheres in a seed alcosol for stober synthesis. J Colloid Interface Sci. 179, 318–321 (1996)
Godoi, R.H.M., Fernandes, L., Jafelicci Jr., M., Marques, R.C., Varanda, L.C., Davolos, M.R.: Investigation of the systems silica and silica containing chromium in alcohol medium. J. Non-Cryst Solids. 247, 141–145 (1999)
Bahadur, H., Srivastava, A.K., Sharma, R.K., Chandra, S.: Morphologies of sol-gel derived thin films of ZnO using different precursor materials and their nanostructures. Nanoscale Res Lett. 2, 469–475 (2007)
Singh, A.K., Viswanath, V., Janu, V.C.C.: Synthesis, effect of capping agents, structural, optical and photoluminescence properties of ZnO nanoparticles. J Lumin. 129, 874–878 (2009)
Ramakrishna, P.V., Murthy, D.B.R.K., Sastry, D.L., Samatha, K.: Synthesis, structural and luminescence properties of Mn doped ZnO/Zn2SiO4 composite microphosphor. Spectrochim Acta A. 129, 274–279 (2014)
Lin, C.C., Shen, P.: Sol-gel synthesis of zinc orthosilicate. J Non-Cryst Solids. 171, 281–289 (1994)
Petrovykh, K.A., Rempel, A.A., Kortov, V.S., Buntov, E.A.: Sol-gel synthesis and photoluminescence of Zn2SiO4:Mn nanoparticles. Inorg Mater. 51(2), 152–157 (2015)
El Ghoul, J., El Mir, L.: Sol-gel synthesis and luminescence of undoped and Mn-doped zinc orthosilicate phosphor nanocomposites. J Lumin. 148, 82–88 (2014)
Taghavinia, N., Lerondel, G., Makino, H., Yamamoto, A., Yao, T., Kawazoe, Y., Goto, T.: Nanocrystalline Zn2SiO4:Mn2+ grown in oxidized porous silicon. Nanotechnology. 12, 547–551 (2001)
Cannas, C., Mainas, M., Musinu, A., Piccaluga, G.: ZnO/SiO2 nanocomposites obtained by impregnation of mesoporous silica. Compos Sci Technol. 63(8), 1187–1191 (2003)
Engku Ali, E.A.G., Matori, K.A., Saion, E., Sidek, H.A.A., Zaid, M.H.M., Alibe, I.M.: Effect of reaction time on structural and optical properties of porous SiO2 nanoparticles. Dig J Nanomater Biostruct. 12, 441–447 (2017)
Omri, K., Ghoul, J.E., Alyamani, A., Barthou, C., Mir, L.E.: Luminescence properties of green emission of SiO2/Zn2SiO4:Mn nanocomposite prepared by sol–gel method. Phys E Low-Dimensional Syst Nanostructures. 53, 48–54 (2013)
Raevskaya, A.E., Panasiuk, Y.V., Stroyuk, O.L., Kuchmiy, S.Y., Dzhagan, V.M., Milekhin, A.G., Yeryukov, N.A., Sveshnikova, L.A., Rodyakina, E.E., Plyusnin, V.F., Zahn, D.R.T.: Spectral and luminescent properties of ZnO-SiO2 core-shell nanoparticles with size-selected ZnO cores. RSC Adv. 4(108), 63393–63401 (2014)
Babu, K.S., Reddy, A.R., Reddy, K.V.: Controlling the size and optical properties of ZnO nanoparticles by capping with SiO2. Mater Res Bull. 49, 537–543 (2014)
Yang, Y., Kim, D.S., Knez, M., Scholz, R., Berger, A., Pippel, E., Hesse, D., Gosele, U., Zacharias, M.: Influence of temperature on evolution of coaxial ZnO/Al2O3 one-dimensional heterostructures: from core-shell nanowires to spinel nanotubes and porous nanowires. J Phys Chem C. 112, 4068–4074 (2008)
Li, Z., Zhang, H., Fu, H.: Facile synthesis and morphology control of Zn2SiO4:Mn nanophosphors using mesoporous silica nanoparticles as templates. J Lumin. 135, 79–83 (2013)
Tomlins, G.W., Routbort, J.L., Mason, T.O.: Oxygen diffusion in single-crystal zinc oxide. J Am Ceram Soc. 81, 869–876 (1998)
Ghoul, J.E., Mir, L.E.: Synthesis by sol–gel process, structural and luminescence of V and Mn doped α-Zn2SiO4. J Mater Sci Mater Electron. 26, 3550–3557 (2015)
Carter, B., Norton, G.: Ceramic materials: science and engineering. Springer Science+Business Media, New York (2007)
El Mir, L., Amlouk, A., Barthou, C., Alaya, S.: Synthesis and luminescence properties of ZnO/Zn2SiO4/SiO2 composite based on nanosized zinc oxide-confined silica aerogels. Phys B Condens Matter. 388, 412–417 (2007)
Ramimoghadam, D., Hussein, M., Taufiq-Yap, Y.: The effect of sodium dodecyl sulfate (SDS) and cetyltrimethylammonium bromide (CTAB) on the properties of ZnO synthesized by hydrothermal method. Int J Mol Sci. 13, 13275–13293 (2012)
Podbršček, P., Dražić, G., Anžlovar, A., Orel, Z.C.: The preparation of zinc silicate/ZnO particles and their use as an efficient UV absorber. Mater Res Bull. 46, 2105–2111 (2011)
Zaid, M.H.M., Matori, K.A., Aziz, S.A., Kamari, H.M., Zaidan, A.W., Fen, Y.W., Alibe, I.M.: Synthesis and characterization of low cost willemite based glass–ceramic for opto-electronic applications. J Mater Sci Mater Electron. 27, 11158–11167 (2016)
Viezbicke, B.D., Patel, S., Davis, B.E., Birnie, D.P.: Evaluation of the Tauc method for optical absorption edge determination: ZnO thin films as a model system. Phys Status Solidi B. (2015). https://doi.org/10.1002/pssb.201552007
Foo, K.L., Hashim, U., Muhammad, K., Voon, C.H.: Sol-gel synthesized zinc oxide nanorods and their structural and optical investigation for optoelectronic application. Nanoscale Res Lett. (2014). https://doi.org/10.1186/1556-276X-9-429
Kumar, S., Sahare, P.D.: Observation of band gap and surface defects of ZnO nanoparticles synthesized via hydrothermal route at different reaction temperature. Opt Commun. 285, 5210–5216 (2012)
Singh, R.G., Singh, F., Mehra, R.M., Kanjilal, D., Agarwal, V.: Synthesis of nanocrystalline α-Zn2SiO4 at ZnO–porous silicon interface: phase transition study. Solid State Commun. 151, 701–703 (2011)
Funding
This study was funded by the Malaysian Ministry of Higher Education (MOHE), Universiti Malaysia Terengganu and Universiti Putra Malaysia through the Inisiatif Putra Berkumpulan (IPB) research grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Engku Ali, E.A.G., Matori, K.A., Saion, E. et al. Effect of sintering temperatures on structural and optical properties of ZnO-Zn2SiO4 composite prepared by using amorphous SiO2 nanoparticles. J Aust Ceram Soc 55, 115–122 (2019). https://doi.org/10.1007/s41779-018-0217-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-018-0217-0