Abstract
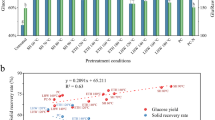
The purpose of this research is to evaluate the anaerobic digestion of apple pomace waste in terms of pretreatment. In this study, the main pretreatment strategies for apple pomace include: ultrasound (35 and 53 kHz), thermal and chemical (pH 5 and 10). For each pretreatment method four different temperatures are selected as 25, 40, 50, and 60 °C, and operation times are selected as 5th, 15th, 30th, and 45th minutes. The effects on pretreatment were investigated by measuring changes in the soluble protein concentrations of pretreated wastes and the enhanced anaerobic digestion was investigated by using the biochemical methane potential (BMP) assay. The soluble proteins of ultrasonic (35 kHz at 60 °C, 45th min), ultrasonic (53 kHz at 60 °C, 45th min), chemical (pH 5 at 60 °C, 5th min), chemical (pH 10 at 60 °C, 30th min) and thermal chemical (40 °C, 15th min) pretreatment apple pomace were 74.3, 75.6, 48.7, 85.5 and 58.6% higher, respectively. The results indicated that apple pomace treated with 53 kHz at 60 °C, 45th min had the highest biogas yield of 1519 mL CH4/g VSS.day after anaerobic digestion, which was on average 40.9% higher than raw pomace.











Similar content being viewed by others
References
APHA (2012) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, DC
Apul OG, Sanin FD (2010) Ultrasonic pre-treatment and subsequent anaerobic digestion under different operational conditions. Bioresour Technol 101(23):8984–8992. https://doi.org/10.1016/j.biortech.2010.06.128
Beszédes S, Kertész S, László Z, Szabó G, Hodúr C (2009) Biogas production of ozone and/or microwave-pretreated canned maize production sludge. Ozone Sci Eng 31(3):257–261. https://doi.org/10.1080/01919510902841218
Cantrell KB, Ducey T, Ro KS, Hunt PG (2008) Livestock waste-to-bioenergy generation opportunities. Bioresour Technol 99:7941–7953. https://doi.org/10.1016/j.biortech.2008.02.061
Cesaro A, Belgiorno V (2014) Pretreatment methods to improve anaerobic biodegradability of organic municipal solid waste fractions. Chem Eng J 240:24–37. https://doi.org/10.1016/j.cej.2013.11.055
Charters WWS (2001) Developing market for renewable energy technologies. Renew Energy 22(1–3):217–222. https://doi.org/10.1016/S0960-1481(00)00018-5
Chen X, Zhang Y, Gu Y, Liu Z, Shen Z, Chu H, Zhou X (2014) Enhancing methane production from rice straw by extrusion pretreatment. Appl Energy 122(1):34–41. https://doi.org/10.1016/j.apenergy.2014.01.076
Choi CH, Um BH, Kim YS, Oh KK (2013) Improved enzyme efficiency of rapeseed straw through the two-stage fractionation process using sodium hydroxide and sulfuric acid. Appl Energy 102:640–646. https://doi.org/10.1016/j.apenergy.2012.08.011
Chynoweth DE, Owens JM, Legrand R (2001) Renewable methane from anaerobic digestion of biomass. Renew Energy 22(1–3):1–8. https://doi.org/10.1016/S0960-1481(00)00019-7
Climent M, Ferrer I, Baeza MD, Artola A, Vazquez F, Font X (2007) Effects of thermal and mechanical pretreatments of secondary sludge on biogas production under thermophilic conditions. Chem Eng J 133(1–3):335–342. https://doi.org/10.1016/j.cej.2007.02.020
Demirbas MF, Balat M (2009) Progress and recent trends in biogas processing. Int J Green Energy 6(2):117–142. https://doi.org/10.1080/15435070902784830
Dhillon GS, Kaur S, Brar SK (2013) Perspective of apple processing wastes as low-cost substrates for bioproduction of high value products: a review. Renew Sustain Energy Rev 27:789–805. https://doi.org/10.1016/j.rser.2013.06.046
Elbeshbishy E, Aldin S, Hafez H, Nakhla G, Ray M (2011) Impact of ultrasonication of hog manure on anaerobic digestibility. Ultrason Sonochem 18(1):164–171. https://doi.org/10.1016/j.ultsonch.2010.04.011
Eskicioglu C, Kennedy KJ, Droste RL (2006) Characterization of soluble organic matter of waste activated sludge before and after thermal pretreatment. Water Resour 40(20):3725–3736. https://doi.org/10.1016/j.watres.2006.08.017
Feng X, Lei H, Deng J, Yu Q, Li H (2009a) Physical and chemical characteristics of waste activated sludge treated ultrasonically. Chem Eng Process 48(1):187–194. https://doi.org/10.1016/j.cep.2008.03.012
Feng X, Deng J, Lei H, Bai T, Fan Q, Li Z (2009b) Dewaterability of waste activated sludge with ultrasound conditioning. Bioresour Technol 100:1074–1081. https://doi.org/10.1016/j.biortech.2008.07.055
Ferreira LC, Donoso-Bravo A, Nilsen PJ, Fdz-Polanco F, Pérez-Elvira SI (2013) Influence of thermal pretreatment on the biochemical methane potential of wheat straw. Bioresour Technol 143:251–257. https://doi.org/10.1016/j.biortech.2013.05.065
Grönroos A, Kyllönen H, Korpijärvi K, Pirkonen P, Paavola T, Jokela J, Rintala J (2005) Ultrasound assisted method to increase soluble chemical oxygen demand (SCOD) of sewage sludge for digestion. Ultrason Sonochem 12(1–2):115–120. https://doi.org/10.1016/j.ultsonch.2004.05.012
Jurado E, Skiadas IV, Gavala HN (2013) Enhanced methane productivity from manure fibers by aqueous ammonia soaking pretreatment. Appl Energy 109:104–111. https://doi.org/10.1016/j.apenergy.2013.03.075
Kafle GK, Kim SH (2013) Anaerobic treatment of apple waste with swine manure for biogas production: batch and continuous operation. Appl Energy 103:61–72. https://doi.org/10.1016/j.apenergy.2012.10.018
Luste S, Luostarinen S, Sillanpää M (2009) Effect of pre-treatments on hydrolysis and methane production potentials of by-products from meat-processing industry. J Hazard Mater 164(1):247–255. https://doi.org/10.1016/j.jhazmat.2008.08.002
Menardo S, Airoldi G, Balsari P (2012) The effect of particle size and thermal pre-treatment on the methane yield of four agricultural by-products. Bioresour Technol 104:708–714. https://doi.org/10.1016/j.biortech.2011.10.061
Muller CD, Abu-Orf M, Blumenschein CD, Novak JT (2009) A comparative study of ultrasonic pretreatment and an internal recycle for the enhancement of mesophilic anaerobic digestion. Water Environ Res 81(12):2398–2410. https://doi.org/10.2175/106143009X407311
Pilli S, Bhunia P, Yan S, LeBlanc RJ, Tyagi RD, Surampalli RY (2011) Ultrasonic pretreatment of sludge: a review. Ultrason Sonochem 18(1):1–18. https://doi.org/10.1016/j.ultsonch.2010.02.014
Rafique R, Poulsen TG, Nizami A, Asam Z, Murphy JD, Kiely G (2010) Effect of thermal, chemical and thermo-chemical pre-treatments to enhance methane production. Energy 35(12):4556–4561. https://doi.org/10.1016/j.energy.2010.07.011
Riggio V, Comino E, Rosso M (2015) Energy production from anaerobic co-digestion processing of cow slurry, olive pomace and apple pulp. Renew Energy 83:1043–1049. https://doi.org/10.1016/j.renene.2015.05.056
Saha M, Eskicioglu C, Marin J (2011) Microwave, ultrasonic and chemomechanical pretreatments for enhancing methane potential of pulp mill wastewater treatment sludge. Bioresour Technol 102(17):7815–7826. https://doi.org/10.1016/j.biortech.2011.06.05
Sterling JMC, Lacey RE, Engler CR, Ricke SC (2001) Effects of ammonia nitrogen on H2 and CH4 production during anaerobic digestion of dairy cattle manure. Bioresour Technol 77(1):9–18. https://doi.org/10.1016/S0960-8524(00)00138-3
TUIK (2015) Turkey Statistics Institution, Ankara, 10752
Vlyssides AG, Karlis PK (2004) Thermal-alkaline solubilization of waste activated sludge as a pre-treatment stage for anaerobic digestion. Bioresour Technol 91(2):201–206. https://doi.org/10.1016/S0960-8524(03)00176-7
Wang Q, Kuninobu M, Kakimoto K, Ogawa HI, Kato Y (1999) Upgrading of anaerobic digestion of waste activated sludge by ultrasonic pre-treatment. Bioresour Technol 68(3):309–313. https://doi.org/10.1016/S0960-8524(98)00155-2
Xu J, Yuan H, Lin J, Yuan W (2014) Evaluation of thermal, thermal-alkaline, alkaline and electrochemical pretreatments on sludge to enhance anaerobic biogas production. J Taiwan Inst Chem E 45(5):2531–2536. https://doi.org/10.1016/j.jtice.2014.05.029
Zhong W, Li Z, Yang J, Liu C, Tian B, Wang Y, Chen P (2014) Effect of thermal–alkaline pretreatment on the anaerobic digestion of streptomycin bacterial residues for methane production. Bioresour Technol 151:436–440. https://doi.org/10.1016/j.biortech.2013.10.100
Zi-lin S, Gai-he Y, Yong-zhong F, Guang-xin R, Xin-hui H (2013) Pretreatment of rice straw by hydrogen peroxide for enhanced methane yield. J Int Agric 12(7):1258–1266. https://doi.org/10.1016/S2095-3119(13)60355-X
Acknowledgements
This work was supported by the Aksaray University Scientific Research Project (2015-018).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tulun, Ş., Bilgin, M. Enhanced Soluble Protein and Biochemical Methane Potential of Apple Biowaste by Different Pretreatment. Earth Syst Environ 2, 85–94 (2018). https://doi.org/10.1007/s41748-017-0033-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41748-017-0033-7