Abstract
ᅟ
Titanium alloys are most often used in the design of dental implants. Although these biomaterials have good mechanical and biological properties, their corrosion resistance is still critical for the overall success of the treatment procedure. Implant failure is more likely to occur in inflammatory diseases related to low pH levels. Er,Cr:YSGG laser is most often used in implant dentistry.
The aim of the this study was to assess the effect of Er,Cr:YSGG laser surface treatment on the corrosion behavior of titanium alloy (Ti–6Al–4V) at different pH.
Materials and methods
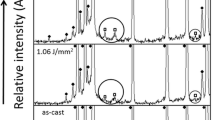
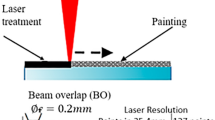
A total of 40 discs were used. Twenty discs were irradiated with Er,Cr:YSGG laser, which was operating in a normal room atmosphere and temperature at power 2 W. The corrosion behavior was investigated in simulated body fluid at pH of 7.40 and 5.20. At each pH, corrosion behavior was studied for up to 864 h at 192, 360, 696, and 864 h intervals using potentiodynamic polarization test and electrochemical impedance spectroscopy. The laser-treated and -untreated discs were examined with scanning electron microscope before and after the electrochemical tests.
Result
Laser treatment significantly improves the corrosion resistance compared to the untreated group. Immersion time and pH also significantly affect the corrosion behavior. The acidity yielded more aggressive changes on the untreated titanium alloy.
Conclusion
Er,Cr:YSGG laser could improve the corrosion resistance of Ti–6Al–4V.




Similar content being viewed by others
References
Piotrowski B, Baptista AA, Patoor E, Bravetti P, Eberhardt A, Laheurte P (2014) Interaction of bone–dental implant with new ultra low modulus alloy using a numerical approach. Mater Sci and Engin C 38:151–160
Triplett RG, Frohberg U, Sykaras N, Woody RD (2003) Implant materials, design and surface topographies: their influence on osseointegration of dental implants. J Long-Term Eff Med Implants 13:485–501
Mathew MT, Abbey S, Hallab NJ, Hall DJ, Sukotjo C, Wimmer MA (2012) Influence of pH on the tribocorrosion behavior of CpTi in the oral environment: synergistic interactions of wear and corrosion. J Biomed Mater Res B Appl Biomater 100:1662–1671
Geetha M, Durgalakshmi D, Asokamani R (2010) Biomedical implants: corrosion and its prevention—a review. Recent Patents Corros Sci 2:40–54
Gimbel CB: (2000) Hard tissue laser procedures. In: Convissar FA, Dent Clin North Am. Philadelphia, PA: Saunders 44:931–948
Featherstone, JD: (2000) Caries detection and prevention with laser energy. In: Convissar FA, Dent Clin North Am. Philadelphia, PA; Saunders 44:955–966
Romanos GE, Gupta B, Yunker M et al (2013) Lasers use in dental implantology. Implant Dent 22:282–288
Matsumoto K, Hossain MM, Kawano H, Kimura Y (2002) Clinical assessment of Er ,Cr: YSGG laser application for cavity preparation. J Clin Laser Med Surg 20:17–21
Arisan V, Karabuda CZ, Ozdemir T (2010) Implant surgery using bone- and mucosa-supported stereolithographic guides in totally edentulous jaws: surgical and post-operative outcomes of computer-aided vs. standard techniques. Clin Oral Implants Res 21(9):980–988
Nickenig H-J, Wichmann M, Schlegel KA, Nkenke E, Eitner S (2010) Radiographic evaluation of marginal bone levels during healing period, adjacent to parallel-screw cylinder implants inserted in the posterior zone of the jaws, placed with flapless surgery. Clin Oral Implants Res 21(12):1386–1393
Gomez-Santos L, Arnabat-Dominguez J, Sierra-Rebolledo A, Gay-Escoda C (2010) Thermal increment due to Er,Cr:YSGG and CO2 laser irradiation of different implant surfaces. Apilot study. Med Oral Patol Oral Cir Bucal 15:782–787
Hansen DC (2008) Metal corrosion in the human body: the ultimate bio-corrosion scenario. Electrochem Soc Interface 17:24–27
Sato N. (2012) Basics of corrosion chemistry.Green corrosion chemistry and engineering 1st edition
Schmutz P, Quach-Vu N, Gerber I (2008) Metallic medical implants: electrochemical characterization of corrosion processes. Electrochem Soc Interface 17:35–40
Al-Mobarak NA, Al-Swayih AA, Al-Rashod FA (2011) Corrosion behavior of Ti-6Al-7Nb alloy in biological solution for dentistry applications. Int J Electrochem Sci 6:2031–2042
Lee JH, Heo SJ, Koak JY, Kim SK, Lee SJ, Lee SH (2008) Cellular responses on anodized titanium discs after laser irradiation. Lasers Surg Med 40:738–742
Neupane MP, Park IS, Lee MH (2014) Surface characterization and corrosion behavior of micro-arc oxidized Ti surface modified with hydrothermal treatment and chitosan coating. Thin Solid Films 550:268–271
Jiang Z, Dai X, Norby T, Middleton H (2011) Investigation of pitting resistance of titanium based on a modified point defect model. Corros Sci 53:815–821
Antunes RA, De Oliveira MCL, Costa I (2012) Study of the correlation between corrosion resistance and semi-conducting properties of the passive film of AISI 316 L stainless steel in physiological solution. Mater Corros 63:586–592
Robin A, Carvalho OAS, Schneider SG, Schneider S. (2008) Corrosion behavior of Ti-xNb-13-Zr alloys in Ringer’s solution, Mater Corrosion 59: 929–933
Guo HX, Lu BT, Luo JL (2006) Non-Faraday material loss in flowing corrosive solution. Electrochim Acta 51:5341–5348
Munirathinam B, Neelakantan L (2015) Titania nanotubes from weak organic acid electrolyte: fabrication, characterization and oxide film properties. Mater Sci Eng C 49:567–578
Park JH, Heo SJ, Koak JY, Kim SK, Han CH, Lee JH (2012) Effects of laser irradiation on machined and anodized titanium disks. Int J Oral Maxillofac Implants 27:265–272
Miller RJ (2004) Treatment of the contaminated implant surface using Er,Cr:YSGG laser. Implant Dent 13:165–170
Li Y, Zhang T, Wang F (2006) Effect of microcrystallization on corrosion resistance of AZ91D alloy. Electrochim Acta 51:2845–2850
Singh R, Kurella A, Dahotre NB (2006) Laser surface modification of Ti-6Al-4V: wear and corrosion characterization in simulated biofluid. J Biomater Appl 21(1):49–73
Biswas A, Li L, Maity TK, Chatterjee UK, Mordike BL, Manna I, Dutta MJ (2007) Laser surface treatment of Ti-6Al-4V for bio-implant application. Lasers Eng 17:59–73
Zaveri N, Mahapatra M, Deceuster A, Peng Y, Li L, Zhou A (2008) Corrosion resistance of pulsed laser-treated Ti-6Al-4V implant in simulated biofluids. Electrochim Acta 53(15):5022–5032
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Abd El daym, D.A., Gheith, M.E., Abbas, N.A. et al. Corrosion behavior of erbium chromium-doped yattrium-scandium-gallium-garnet (Er,Cr:YSGG 2780 nm) laser-treated titanium alloy used for dental applications at different pH conditions (in vitro study). Laser Dent Sci 2, 137–146 (2018). https://doi.org/10.1007/s41547-018-0030-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41547-018-0030-7