Abstract
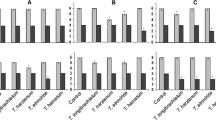
Albugo candida, the causative agent of white rust disease in Indian mustard [Brassica juncea (L.) Czern. & Coss.], affects yield and quality of the crops. Biocontrol agents that induce disease resistance in crop plants against pathogen could be a reliable alternative to the chemical control. In this regard, we demonstrated the capabilities of Trichoderma harzianum, an efficient biocontrol agent to induce resistance in B. juncea against A. candida under green house conditions. Trichoderma harzianum treatment of Brassica seedlings followed by A. candida infection demonstrated an acquired resistance with an optimal disease control (45.6% disease reduction). This interaction exhibited an increase in antioxidant enzymes (glutathione reductase and peroxidase) and decreased in phosphatase activity. It also induced up-regulation of MAPK 6 and 17 genes. Hence, it is concluded that T. harzianum induced disease resistance in B. juncea against A. candida infection through the involvement of MAP kinase and up-regulation of antioxidant enzymes.







Similar content being viewed by others
References
Alkooranee JT, Yin Y, Aledan TR, Jiang Y, Lu G, Wu J et al (2015) Systemic resistance to powdery mildew in Brassica napus (AACC) and Raphanus alboglabra (RRCC) by Trichoderma harzianum TH12. PLoS ONE 10(11):e0142177
Bergmeyer HU (1974) Method of enzymatic analysis. Academic Press, New York
Błaszczyk L, Siwulski M, Sobieralski K, Lisiecka J, Jędryczka M (2014) Trichoderma spp. application and prospects for use in organic farming and industry. J Plant Prot Res 54(4):309–317
Bock CH, Poole GH, Parker PE, Gottwald TR (2010) Plant disease severity estimated visually, by digital photography and image analysis, and by hyperspectral imaging. Crit Rev Plant Sci 29(2):59–107
Bolwell PP, Page A, Piślewska M, Wojtaszek P (2001) Pathogenic infection and the oxidative defences in plant apoplast. Protoplasma 217(1–3):20–32
Chakraborty MR, Chatterjee NC (2007) Interaction of Trichoderma harzianum with Fusarium solani during its Pathogenesis and the associated resistance of the host. Asian J Exp Sci 21(2):351–355
Chet Y Henis (1981) A selective medium for improving quantitative isolation of Trichoderma spp. from soil. Phytoparasitica 9:59–67
Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J et al (2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17(1):268–281
Deng S, Lorito M, Penttila M, Harman GE (2007) Over expression of an endochitinase gene (ThEn-42) in Trichoderma atroviride for increased production of antifungal enzymes and enhanced antagonist action against pathogenic fungi. Appl Biochem Biotechnol 142:81–94
Druzhinina IS, Seidl-Seiboth V, Herrera-Estrella A, Horwitz BA, Kenerley CM, Monte E et al (2011) Trichoderma: the genomics of opportunistic success. Nat Rev Microbiol 9(10):749–759
El-Mohamedy RSR (2009) Efficiency of different application methods of biocontrol agents and biocides in control of Fusarium root rot on some citrus rootstocks. Arch Phytopathol Plant Prot 42(9):819–828
Feechan A, Kwon E, Yun BW, Wang Y, Pallas JA, Loake GJ (2005) A central role for S-nitrosothiols in plant disease resistance. Proc Natl Acad Sci USA 102(22):8054–8059
Gajera HP, Savaliya DD, Patel SV, Golakiya BA (2015) Trichoderma viride induces pathogenesis related defence response against rot pathogen infection in groundnut (Arachis hypogaea L.). Infect Genet Evolut 34:314–325
Gao J, Cao M, Ye W, Li H, Kong L, Zheng X et al (2015) PsMPK7, a stress-associated mitogen-activated protein kinase (MAPK) in Phytophthora sojae, is required for stress tolerance, reactive oxygenated species detoxification, cyst germination, sexual reproduction and infection of soybean. Mol Plant Pathol 16(1):61–70
Gill SS, Anjum NA, Hasanuzzaman M, Gill R, Trivedi DK, Ahmad I et al (2013) Glutathione and glutathione reductase: a boon in disguise for plant abiotic stress defence operations. Plant Physiol Biochem 70:204–212
Hahn MG (1996) Microbial elicitors and their receptors in plants. Annu Rev Phytopathol 34:387–412
Harel YM, Mehari ZH, Rav-David D, Elad Y (2014) Systemic resistance to gray mold induced in tomato by benzothiadiazole and Trichoderma harzianum T39. Phytopathology 104(2):150–157
Harman GE (2006) Overview of mechanisms and uses of Trichoderma spp. Phytopathology 96(2):190–194
Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species–opportunistic, avirulent plant symbionts. Nat Rev Microbiol 2(1):43–56
Hermosa R, Viterbo A, Chet I, Monte E (2012) Plant-beneficial effects of Trichoderma and of its genes. Microbiology 158(Pt 1):17–25
Hoitink HA, Madden LV, Dorrance AE (2006) Systemic resistance induced by Trichoderma spp.: interactions between the host, the pathogen, the biocontrol agent, and soil organic matter quality. Phytopathology 96(2):186–189
Howell CR (2003) Mechanisms employed by Trichoderma species in the biological control of plant disease: the history and evolution of current concepts. Plant Dis 87:4–10
Howell CR, Hanson LE, Stipanovic RD, Puckhaber LS (2000) Induction of terpenoid synthesis in cotton roots and control of Rhizoctonia solani by seed treatment with Trichoderma virens. Phytopathology 90:248–252
Keyse SM (2000) Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol 12(2):186–192
Khaledi N, Taheri P (2016) Biocontrol mechanisms of Trichoderma harzianum against soybean charcoal rot caused by Macrophomina phaseolina. J Plant Prot Res 56(1):21–31
Kumar P, Kumar C (2013) Trichoderma induce alteration of serine/threonine and tyrosine phosphatase in bipartite interaction of Brassica juncea. Int J Bioinf Biol Sci 1(2):165–172
Lamdan NL, Shalaby S, Ziv T, Kenerley CM, Horwitz BA (2015) Secretome of Trichoderma interacting with maize roots: role in induced systemic resistance. Mol Cell Proteomics 14(4):1054–1063
Lewis JD (2016) Plant immunity. Semin Cell Dev Biol 56:122–123
Marra R, Ambrosino P, Carbone V, Vinale F, Woo SL, Ruocco M et al (2006) Study of the three-way interaction between Trichoderma atroviride, plant and fungal pathogens by using a proteomic approach. Curr Genet 50:307–321
Mastouri F, Björkman T, Harman GE (2012) Trichoderma harzianum enhances antioxidant defense of tomato seedlings and resistance to water deficit. Mol Plant Microbe Interact 25(9):1264–1271
Miguel E, Poza-Carrión C, López-Solanilla E, Aguilar I, Llama-Palacios A, García-Olmedo F et al (2000) Evidence against a direct antimicrobial role of H2O2 in the infection of plants by Erwinia chrysanthemi. Mol Plant Microbe Interact 13(4):421–429
Mukherjee PK, Latha J, Hadar R, Horwitz BA (2003) TmkA, a MAP kinase of Trichoderma virens is involved in biocontrol properties and repression of condition in dark. Eukaryot Cell 2:446–455
Perazzolli M, Moretto M, Fontana P, Ferrarini A, Velasco R, Moser C et al (2012) Downy mildew resistance induced by Trichoderma harzianum T39 in susceptible grapevines partially mimics transcriptional changes of resistant genotypes. BMC Genom 13:660
Saharan GS, Verma PR, Meena PD, Kumar A (2014) White rust of crucifers: biology, ecology and management. Springer, New Delhi
Shoresh M, Gal-On A, Leibman D, Chet I (2006) Characterization of a mitogen-activated protein kinase gene from cucumber required for Trichoderma-conferred plant resistance. Plant Physiol 142(3):1169–1179
Shoresh M, Harman GE, Mastouri F (2010) Induced systemic resistance and plant responses to fungal biocontrol agents. Annu Rev Phytopathol 48:21–43
Singh BN, Singh A, Singh BR, Singh HB (2014) Trichoderma harzianum elicits induced resistance in sunflower challenged by Rhizoctonia solani. J Appl Microbiol 116(3):654–666
Smith IK, Vierheller TL, Thorne CA (1998) Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid). Anal Biochem 175(2):408–413
Sreedhar HS, Shetty SA, Shetty HS (1995) Peroxidase activities in pathogenesis of Sclerospora graminicola in pearl millet seedlings. Int J Trop Plant Dis 13(1):19–32
Theodosiou A, Ashworth A (2002) MAP kinase phosphatases. Genome Biol 3(7):reviews3009
Tucci M, Ruocco M, De Masi L, De Palma M, Lorito M (2011) The beneficial effect of Trichoderma sp. on tomato is modulated by the plant genotype. Mol Plant Pathol 12(4):341–354
Vilela B, Pagès M, Lumbreras V (2010) Regulation of MAPK signaling and cell death by MAPK phosphatase MKP2. Plant Signal Behav 5(11):1497–1500
Vinale F, Sivasithamparam K, Ghisalberti EL, Marra R, Woo SL, Lorito M (2008) Trichoderma-plant-pathogen interactions. Soil Biol Biochem 40:1–10
Viterbo A, Harel M, Horwitz DA, Chet I, Mukherjee PK (2005) Trichoderma MAP Kinase signaling is involved in induction of plant systemic resistance. Appl Environ Microbiol 71:6241–6246
Woo SL, Scala F, Rocco M, Lorito M (2006) The molecular biology of the interactions between Trichoderma sp., phytopathogenic fungi, and plants. Phytopathology 96(2):181–185
Yang DL, Yang Y, He Z (2013) Roles of plant hormones and their interplay in rice immunity. Mol Plant 6(3):675–685
Yedidia I, Benhamou N, Chet I (1999) Induction of defence responses in cucumber plants (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Appl Environ Microbiol 65:1061–1070
Yedidia I, Benhamoub N, Yoram KY, Chet I (2000) Induction and accumulation of PR proteins activity during early stages of root colonization by the mycoparasite Trichoderma harzianum strain T-203. Plant Physiol Biochem 38:863–873
Zeilinger S, Omann M (2007) Trichoderma biocontrol: signal transduction pathways involved in host sensing and mycoparasitism. Gene Regul Syst Biol 8(1):227–234
Acknowledgements
The first author wishes to thank Department of Biotechnology (DBT), India, for financial support and Bhabha Atomic Research Centre (BARC) for providing facilities for carrying out research. The first author is thankful to the Dr. U. S. Singh and Dr. Anil K. Gupta GBPUA&T, Pantnagar, India, for providing the facilities necessary to conduct our initial research work. We are also thankful to Dr. (Mrs.) Meera Venkatesh and Dr. (Mrs.) Grace Samuel of Radiopharmaceuticals Division, BARC, for providing laboratory facilities and auxiliary staff to carry out radioactive experiments and molecular biology work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, P., Kumar, C. Molecular and enzymatic approach to study Trichoderma harzianum-induced disease resistance in Brassica juncea against Albugo candida . J Plant Dis Prot 125, 167–175 (2018). https://doi.org/10.1007/s41348-017-0137-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-017-0137-1