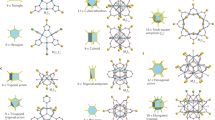
Abstract
The opportunity to generate functional solids with defined properties by deliberate design has not been materialized in traditional solid-state chemistry over many decades. The emergence of metal–organic frameworks (MOFs), permanently porous, crystalline solids with defined metrics, has allowed for studying design, synthesis, and properties, which then translated into new applications. Aggregates of metal ions stitched together by multidentate functional groups form such metal oxide clusters and represent the nodes of MOFs. These clusters, termed secondary building units (SBUs), are decorated with organic moieties that provide directionality and can be linked through geometric principles into extended nets using organic molecules (spacers). This concept of reticular chemistry has afforded permanently porous MOFs, and has resulted in over 20,000 structures over the past 20 years. However, there are still only a limited number of symmetric, discrete SBUs commonly used to design and synthesize MOFs. We herein introduce the most important SBUs that have emerged over time together with prototypal MOF structures and their fundamental applications. Both the discovery and the scientific impact will be highlighted alongside advantages and/or drawbacks. In addition, an outlook will be given on how the combination of multiple SBUs can lead to heterogeneous but ordered materials with higher complexity and functionality.



















Similar content being viewed by others
References
Maddox J (1988) Crystals from first principles. Nature 335:201
Hoskins BF, Robson R (1989) Infinite polymeric frameworks consisting of three dimensionally linked rod-like segments. J Am Chem Soc 111(15):5962–5964. https://doi.org/10.1021/ja00197a079
Kinoshita Y, Matsubara I, Saito Y (1959) The crystal structure of bis(glutaronitrilo)copper(I) nitrate. Bull Chem Soc Jpn 32 (11):1216–1221. https://www.jstage.jst.go.jp/article/bcsj1926/32/11/32_11_1216/_pdf
Kinoshita Y, Matsubara I, Saito Y (1959) The crystal structure of bis(succinonitrilo)copper(I) nitrate. Bull Chem Soc Jpn 32 (7):741–747. https://www.jstage.jst.go.jp/article/bcsj1926/32/7/32_7_741/_pdf
Kinoshita Y, Matsubara I, Higuchi T, Saito Y (1959) The crystal structure of bis(adiponitrilo)copper(I) nitrate. Bull Chem Soc Jpn 32(11):1221–1226. https://www.jstage.jst.go.jp/article/bcsj1926/32/11/32_11_1221/_pdf
Nugent P, Belmabkhout Y, Burd SD, Cairns AJ, Luebke R, Forrest K, Pham T, Ma S, Space B, Wojtas L, Eddaoudi M, Zaworotko MJ (2013) Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 495(7439):80–84
Adil K, Belmabkhout Y, Pillai RS, Cadiau A, Bhatt PM, Assen AH, Maurin G, Eddaoudi M (2017) Gas/vapour separation using ultra-microporous metal–organic frameworks: insights into the structure/separation relationship. Chem Soc Rev 46(11):3402–3430. https://doi.org/10.1039/C7CS00153C
Schoedel A, Ji Z, Yaghi OM (2016) The role of metal–organic frameworks in a carbon-neutral energy cycle. Nature Energy 1:16034. https://doi.org/10.1038/nenergy.2016.34. http://www.nature.com/articles/nenergy201634#supplementary-information
Eddaoudi M, Moler DB, Li H, Chen B, Reineke TM, O’Keeffe M, Yaghi OM (2001) Modular chemistry: secondary building units as a basis for the design of highly porous and robust metal−organic carboxylate frameworks. Acc Chem Res 34(4):319–330. https://doi.org/10.1021/ar000034b
Moulton B, Zaworotko MJ (2001) From molecules to crystal engineering: supramolecular isomerism and polymorphism in network solids. Chem Rev 101(6):1629–1658. https://doi.org/10.1021/cr9900432
Yaghi OM, O’Keeffe M, Ockwig NW, Chae HK, Eddaoudi M, Kim J (2003) Reticular synthesis and the design of new materials. Nature 423(6941):705
Furukawa H, Cordova KE, O’Keeffe M, Yaghi OM (2013) The chemistry and applications of metal–organic frameworks. Science 341(6149):1230444
Baerlocher C, McCusker LB, Olson DH (2007) Atlas of zeolite framework types, 6th revised edn. Elsevier Science, Amsterdam
Zhao D, Feng J, Huo Q, Melosh N, Fredrickson GH, Chmelka BF, Stucky GD (1998) Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 Angstrom pores. Science 279(5350):548–552
Zakhidov AA, Baughman RH, Iqbal Z, Cui C, Khayrullin I, Dantas SO, Marti J, Ralchenko VG (1998) Carbon structures with three-dimensional periodicity at optical wavelengths. Science 282(5390):897–901
Jiang J, Zhao Y, Yaghi OM (2016) Covalent chemistry beyond molecules. J Am Chem Soc 138(10):3255–3265. https://doi.org/10.1021/jacs.5b10666
Li H, Eddaoudi M, Groy TL, Yaghi OM (1998) Establishing microporosity in open metal−organic frameworks: gas sorption isotherms for Zn(BDC) (BDC = 1,4-benzenedicarboxylate). J Am Chem Soc 120(33):8571–8572. https://doi.org/10.1021/ja981669x
Chui SSY, Lo SMF, Charmant JPH, Orpen AG, Williams ID (1999) A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science 283(5405):1148–1150. https://doi.org/10.1126/science.283.5405.1148
Kondo M, Yoshitomi T, Matsuzaka H, Kitagawa S, Seki K (1997) Three-dimensional framework with channeling cavities for small molecules: {[M2(4,4′-bpy)3(NO3)4]·xH2O}n (M = Co, Ni, Zn). Angew Chem Int Ed Engl 36(16):1725–1727. https://doi.org/10.1002/anie.199717251
Tranchemontagne DJ, Mendoza-Cortés JL, O’Keeffe M, Yaghi OM (2009) Secondary building units, nets and bonding in the chemistry of metal–organic frameworks. Chem Soc Rev 38(5):1257. https://doi.org/10.1039/b817735j
Deng H, Doonan CJ, Furukawa H, Ferreira RB, Towne J, Knobler CB, Wang B, Yaghi OM (2010) Multiple functional groups of varying ratios in metal–organic frameworks. Science 327(5967):846–850. https://doi.org/10.1126/science.1181761
O’Keeffe M, Peskov MA, Ramsden SJ, Yaghi OM (2008) The Reticular Chemistry Structure Resource (RCSR) database of, and symbols for, crystal nets. Acc Chem Res 41 (12):1782–1789. http://rcsr.net. https://doi.org/10.1021/ar800124u
O’Keeffe M, Yaghi OM (2012) Deconstructing the crystal structures of metal–organic frameworks and related materials into their underlying nets. Chem Rev 112(2):675–702. https://doi.org/10.1021/cr200205j
Eddaoudi M, Kim J, Rosi N, Vodak D, Wachter J, O’Keeffe M, Yaghi OM (2002) Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 295(5554):469–472. https://doi.org/10.1126/science.1067208
Fankuchen I (1935) Crystal structure of sodium uranyl acetate. Cryst Mater. https://doi.org/10.1524/zkri.1935.91.1.473
Go YB, Wang X, Jacobson AJ (2007) (6,3)-Honeycomb structures of uranium(VI) benzenedicarboxylate derivatives: the use of noncovalent interactions to prevent interpenetration. Inorg Chem 46(16):6594–6600. https://doi.org/10.1021/ic700693f
Wang Y, Liu Z, Li Y, Bai Z, Liu W, Wang Y, Xu X, Xiao C, Sheng D, Diwu J, Su J, Chai Z, Albrecht-Schmitt TE, Wang S (2015) Umbellate distortions of the uranyl coordination environment result in a stable and porous polycatenated framework that can effectively remove cesium from aqueous solutions. J Am Chem Soc 137(19):6144–6147. https://doi.org/10.1021/jacs.5b02480
Hu K-Q, Jiang X, Wang C-Z, Mei L, Xie Z-N, Tao W-Q, Zhang X-L, Chai Z-F, Shi W-Q (2016) Solvent-dependent synthesis of porous anionic uranyl-organic frameworks featuring a highly symmetrical (3,4)-connected ctn or bor topology for selective dye adsorption. Chem Eur J 23(3):529–532. https://doi.org/10.1002/chem.201604225
Liu C, Chen F-Y, Tian H-R, Ai J, Yang W, Pan Q-J, Sun Z-M (2017) Interpenetrated uranyl-organic frameworks with bor and pts topology: structure, spectroscopy, and computation. Inorg Chem 56(22):14147–14156. https://doi.org/10.1021/acs.inorgchem.7b02274
Li P, Vermeulen NA, Malliakas CD, Gómez-Gualdrón DA, Howarth AJ, Mehdi BL, Dohnalkova A, Browning ND, O’Keeffe M, Farha OK (2017) Bottom-up construction of a superstructure in a porous uranium-organic crystal. Science. https://doi.org/10.1126/science.aam7851
Li P, Vermeulen NA, Gong X, Malliakas CD, Stoddart JF, Hupp JT, Farha OK (2016) Design and synthesis of a water-stable anionic uranium-based metal–organic framework (MOF) with ultra large pores. Angew Chem Int Ed 55(35):10358–10362. https://doi.org/10.1002/anie.201605547
Furukawa H, Go YB, Ko N, Park YK, Uribe-Romo FJ, Kim J, O’Keeffe M, Yaghi OM (2011) Isoreticular expansion of metal–organic frameworks with triangular and square building units and the lowest calculated density for porous crystals. Inorg Chem 50(18):9147–9152. https://doi.org/10.1021/ic201376t
Chen B, Eddaoudi M, Reineke TM, Kampf JW, O’Keeffe M, Yaghi OM (2000) Cu2(ATC)·6H2O: design of open metal sites in porous metal−organic crystals (ATC: 1,3,5,7-adamantane tetracarboxylate). J Am Chem Soc 122(46):11559–11560. https://doi.org/10.1021/ja003159k
Ma L, Jin A, Xie Z, Lin W (2009) Freeze drying significantly increases permanent porosity and hydrogen uptake in 4,4-connected metal–organic frameworks. Angew Chem Int Ed 48(52):9905–9908. https://doi.org/10.1002/anie.200904983
Chen B, Eddaoudi M, Hyde ST, O’Keeffe M, Yaghi OM (2001) Interwoven metal–organic framework on a periodic minimal surface with extra-large pores. Science 291(5506):1021–1023
Eddaoudi M, Kim J, O’Keeffe M, Yaghi OM (2002) Cu2[o-Br-C6H3(CO2)2]2(H2O)2·(DMF)8(H2O)2: a framework deliberately designed to have the NbO structure type. J Am Chem Soc 124(3):376–377. https://doi.org/10.1021/ja017154e
Eddaoudi M, Kim J, Vodak D, Sudik A, Wachter J, O’Keeffe M, Yaghi OM (2002) Geometric requirements and examples of important structures in the assembly of square building blocks. Proc Natl Acad Sci USA 99(8):4900–4904
Moulton B, Lu J, Hajndl R, Hariharan S, Zaworotko MJ (2002) Crystal engineering of a nanoscale Kagomé lattice. Angew Chem Int Ed 41(15):2821–2824. https://doi.org/10.1002/1521-3773(20020802)41:15%3c2821:aid-anie2821%3e3.0.co;2-y
Chen B, Ockwig NW, Millward AR, Contreras DS, Yaghi OM (2005) High H2 adsorption in a microporous metal–organic framework with open metal sites. Angew Chem Int Ed 44(30):4745–4749
Duong TD, Sapchenko SA, da Silva I, Godfrey HGW, Cheng Y, Daemen LL, Manuel P, Ramirez-Cuesta AJ, Yang S, Schröder M (2018) Optimal binding of acetylene to a nitro-decorated metal–organic framework. J Am Chem Soc. https://doi.org/10.1021/jacs.8b08504
Eddaoudi M, Kim J, Wachter JB, Chae HK, O’Keeffe M, Yaghi OM (2001) Porous metal−organic polyhedra: 25 Å cuboctahedron constructed from 12 Cu2(CO2)4 paddle-wheel building blocks. J Am Chem Soc 123(18):4368–4369. https://doi.org/10.1021/ja0104352
Nouar F, Eubank JF, Bousquet T, Wojtas L, Zaworotko MJ, Eddaoudi M (2008) Supermolecular building blocks (SBBs) for the design and synthesis of highly porous metal–organic frameworks. J Am Chem Soc 130(6):1833–1835. https://doi.org/10.1021/ja710123s
Guillerm V, Kim D, Eubank JF, Luebke R, Liu X, Adil K, Lah MS, Eddaoudi M (2014) A supermolecular building approach for the design and construction of metal–organic frameworks. Chem Soc Rev 43(16):6141–6172. https://doi.org/10.1039/c4cs00135d
Zheng B, Bai J, Duan J, Wojtas L, Zaworotko MJ (2010) Enhanced CO2 binding affinity of a high-uptake rht-type metal−organic framework decorated with acylamide groups. J Am Chem Soc 133(4):748–751. https://doi.org/10.1021/ja110042b
Zhao D, Yuan D, Sun D, Zhou H-C (2009) Stabilization of metal−organic frameworks with high surface areas by the incorporation of mesocavities with microwindows. J Am Chem Soc 131(26):9186–9188. https://doi.org/10.1021/ja901109t
Farha OK, Özgür Yazaydın A, Eryazici I, Malliakas CD, Hauser BG, Kanatzidis MG, Nguyen ST, Snurr RQ, Hupp JT (2010) De novo synthesis of a metal–organic framework material featuring ultrahigh surface area and gas storage capacities. Nat Chem 2(11):944–948
Yuan D, Zhao D, Sun D, Zhou H-C (2010) An isoreticular series of metal–organic frameworks with dendritic hexacarboxylate ligands and exceptionally high gas-uptake capacity. Angew Chem Int Ed 49(31):5357–5361. https://doi.org/10.1002/anie.201001009
Farha OK, Eryazici I, Jeong NC, Hauser BG, Wilmer CE, Sarjeant AA, Snurr RQ, Nguyen ST, Yazaydın AÖ, Hupp JT (2012) Metal–organic framework materials with ultrahigh surface areas: is the sky the limit? J Am Chem Soc 134(36):15016–15021. https://doi.org/10.1021/ja3055639
Li J-R, Timmons DJ, Zhou H-C (2009) Interconversion between molecular polyhedra and metal−organic frameworks. J Am Chem Soc 131(18):6368–6369. https://doi.org/10.1021/ja901731z
Stoeck U, Krause S, Bon V, Senkovska I, Kaskel S (2012) A highly porous metal–organic framework, constructed from a cuboctahedral super-molecular building block, with exceptionally high methane uptake. Chem Commun 48(88):10841–10843. https://doi.org/10.1039/c2cc34840c
Stoeck U, Senkovska I, Bon V, Krause S, Kaskel S (2015) Assembly of metal–organic polyhedra into highly porous frameworks for ethene delivery. Chem Commun 51(6):1046–1049. https://doi.org/10.1039/c4cc07920e
Abourahma H, Bodwell GJ, Lu J, Moulton B, Pottie IR, Walsh RB, Zaworotko MJ (2003) Coordination polymers from calixarene-like [Cu2(dicarboxylate)2]4 building blocks: structural diversity via atropisomerism. Cryst Growth Des 3(4):513–519. https://doi.org/10.1021/cg0340345
Tan C, Yang S, Champness NR, Lin X, Blake AJ, Lewis W, Schroder M (2011) High capacity gas storage by a 4,8-connected metal–organic polyhedral framework. Chem Commun 47(15):4487–4489
Li M, Li D, O’Keeffe M, Yaghi OM (2014) Topological analysis of metal–organic frameworks with polytopic linkers and/or multiple building units and the minimal transitivity principle. Chem Rev 114(2):1343–1370. https://doi.org/10.1021/cr400392k
Pang J, Jiang F, Wu M, Liu C, Su K, Lu W, Yuan D, Hong M (2015) A porous metal–organic framework with ultrahigh acetylene uptake capacity under ambient conditions. Nat Commun 6:7575. https://doi.org/10.1038/ncomms8575
Rao X, Cai J, Yu J, He Y, Wu C, Zhou W, Yildirim T, Chen B, Qian G (2013) A microporous metal–organic framework with both open metal and Lewis basic pyridyl sites for high C2H2 and CH4 storage at room temperature. Chem Commun 49(60):6719–6721. https://doi.org/10.1039/c3cc41866a
Cavka JH, Jakobsen S, Olsbye U, Guillou N, Lamberti C, Bordiga S, Lillerud KP (2008) A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J Am Chem Soc 130(42):13850–13851. https://doi.org/10.1021/ja8057953
Reinsch H, Stassen I, Bueken B, Lieb A, Ameloot R, De Vos D (2015) First examples of aliphatic zirconium MOFs and the influence of inorganic anions on their crystal structures. CrystEngComm 17(2):331–337. https://doi.org/10.1039/C4CE01457J
Zhang Y, Zhang X, Lyu J, Otake K-i, Wang X, Redfern LR, Malliakas CD, Li Z, Islamoglu T, Wang B, Farha OK (2018) A flexible metal–organic framework with 4-connected Zr6 nodes. J Am Chem Soc 140(36):11179–11183. https://doi.org/10.1021/jacs.8b06789
Sarkisov L, Martin RL, Haranczyk M, Smit B (2014) On the flexibility of metal–organic frameworks. J Am Chem Soc 136(6):2228–2231. https://doi.org/10.1021/ja411673b
Koyama H, Saito Y (1954) The crystal structure of zinc oxyacetate, Zn4O(CH3COO)6. Bull Chem Soc Jpn 27(2):112–114. https://doi.org/10.1246/bcsj.27.112
Li H, Eddaoudi M, O’Keeffe M, Yaghi OM (1999) Design and synthesis of an exceptionally stable and highly porous metal–organic framework. Nature 402(6759):276–279
Koh K, Van Oosterhout JD, Roy S, Wong-Foy AG, Matzger AJ (2012) Exceptional surface area from coordination copolymers derived from two linear linkers of differing lengths. Chem Sci 3(8):2429–2432
Wong-Foy AG, Matzger AJ, Yaghi OM (2006) Exceptional H2 saturation uptake in microporous metal−organic frameworks. J Am Chem Soc 128(11):3494–3495. https://doi.org/10.1021/ja058213h
Chae HK, Siberio-Perez DY, Kim J, Go Y, Eddaoudi M, Matzger AJ, O’Keeffe M, Yaghi OM (2004) A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 427(6974):523–527
Furukawa H, Ko N, Go YB, Aratani N, Choi SB, Choi E, Yazaydin AÖ, Snurr RQ, O’Keeffe M, Kim J, Yaghi OM (2010) Ultrahigh porosity in metal–organic frameworks. Science 329(5990):424–428
Klein N, Senkovska I, Gedrich K, Stoeck U, Henschel A, Mueller U, Kaskel S (2009) A mesoporous metal–organic framework. Angew Chem Int Ed 48(52):9954–9957. https://doi.org/10.1002/anie.200904599
Liu L, Konstas K, Hill MR, Telfer SG (2013) Programmed pore architectures in modular quaternary metal–organic frameworks. J Am Chem Soc 135(47):17731–17734. https://doi.org/10.1021/ja4100244
Hönicke IM, Senkovska I, Bon V, Baburin IA, Bönisch N, Raschke S, Evans JD, Kaskel S (2018) Balancing mechanical stability and ultrahigh porosity in crystalline framework materials. Angew Chem Int Ed 57(42):13780–13783. https://doi.org/10.1002/anie.201808240
Tu B, Pang Q, Ning E, Yan W, Qi Y, Wu D, Li Q (2015) Heterogeneity within a mesoporous metal–organic framework with three distinct metal-containing building units. J Am Chem Soc 137(42):13456–13459. https://doi.org/10.1021/jacs.5b07687
Tu B, Pang Q, Xu H, Li X, Wang Y, Ma Z, Weng L, Li Q (2017) Reversible redox activity in multicomponent metal–organic frameworks constructed from trinuclear copper pyrazolate building blocks. J Am Chem Soc 139(23):7998–8007. https://doi.org/10.1021/jacs.7b03578
Figgis BN, Robertson GB (1965) Crystal-molecular structure and magnetic properties of Cr3(CH3.COO)6 O Cl.5H2O. Nature 205(4972):694–695
Barthelet K, Riou D, Férey G (2002) [VIII(H2O)]3O(O2CC6H4CO2)3·(Cl, 9H2O) (MIL-59): a rare example of vanadocarboxylate with a magnetically frustrated three-dimensional hybrid framework. Chem Commun 14:1492–1493
Mellot-Draznieks C, Serre C, Surblé S, Audebrand N, Férey G (2005) Very large swelling in hybrid frameworks: a combined computational and powder diffraction study. J Am Chem Soc 127(46):16273–16278. https://doi.org/10.1021/ja054900x
Sudik AC, Côté AP, Yaghi OM (2005) Metal–organic frameworks based on trigonal prismatic building blocks and the new “acs” topology. Inorg Chem 44(9):2998–3000. https://doi.org/10.1021/ic050064g
Serre C, Millange F, Surblé S, Férey G (2004) A route to the synthesis of trivalent transition-metal porous carboxylates with trimeric secondary building units. Angew Chem Int Ed 43(46):6285–6289
Férey G, Mellot-Draznieks C, Serre C, Millange F, Dutour J, Surblé S, Margiolaki I (2005) A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 309(5743):2040–2042
Surblé S, Serre C, Mellot-Draznieks C, Millange F, Férey G (2006) A new isoreticular class of metal–organic-frameworks with the MIL-88 topology. Chem Commun 3:284. https://doi.org/10.1039/b512169h
Serre C, Mellot-Draznieks C, Surblé S, Audebrand N, Filinchuk Y, Férey G (2007) Role of solvent–host interactions that lead to very large swelling of hybrid frameworks. Science 315(5820):1828–1831
Shen J-Q, Liao P-Q, Zhou D-D, He C-T, Wu J-X, Zhang W-X, Zhang J-P, Chen X-M (2017) Modular and stepwise synthesis of a hybrid metal–organic framework for efficient electrocatalytic oxygen evolution. J Am Chem Soc 139(5):1778–1781. https://doi.org/10.1021/jacs.6b12353
Mellot-Draznieks C, Dutour J, Férey G (2004) Computational design of hybrid frameworks: structure and energetics of two Me3OF3{–O2C–C6H4–CO2–}3 metal-dicarboxylate polymorphs, MIL-hypo-1 and MIL-hypo-2. Z Anorg Allg Chem 630(15):2599–2604. https://doi.org/10.1002/zaac.200400416
Liu Y, Eubank JF, Cairns AJ, Eckert J, Kravtsov VC, Luebke R, Eddaoudi M (2007) Assembly of metal–organic frameworks (MOFs) based on indium-trimer building blocks: a porous MOF with soc topology and high hydrogen storage. Angew Chem Int Ed 46(18):3278–3283
Feng D, Wang K, Wei Z, Chen Y-P, Simon CM, Arvapally RK, Martin RL, Bosch M, Liu T-F, Fordham S, Yuan D, Omary MA, Haranczyk M, Smit B, Zhou H-C (2014) Kinetically tuned dimensional augmentation as a versatile synthetic route towards robust metal–organic frameworks. Nat Commun. https://doi.org/10.1038/ncomms6723
Alezi D, Belmabkhout Y, Suyetin M, Bhatt PM, Weseliński ŁJ, Solovyeva V, Adil K, Spanopoulos I, Trikalitis PN, Emwas A-H, Eddaoudi M (2015) MOF crystal chemistry paving the way to gas storage needs: aluminum-based soc-MOF for CH4, O2, and CO2 storage. J Am Chem Soc 137(41):13308–13318. https://doi.org/10.1021/jacs.5b07053
Towsif Abtab SM, Alezi D, Bhatt PM, Shkurenko A, Belmabkhout Y, Aggarwal H, Weseliński ŁJ, Alsadun N, Samin U, Hedhili MN, Eddaoudi M (2018) Reticular chemistry in action: a hydrolytically stable MOF capturing twice its weight in adsorbed water. Chem 4(1):94–105. https://doi.org/10.1016/j.chempr.2017.11.005
Wang K, Feng D, Liu T-F, Su J, Yuan S, Chen Y-P, Bosch M, Zou X, Zhou H-C (2014) A series of highly stable mesoporous metalloporphyrin Fe-MOFs. J Am Chem Soc 136(40):13983–13986. https://doi.org/10.1021/ja507269n
Feng D, Wang K, Wei Z, Chen Y-P, Simon CM, Arvapally RK, Martin RL, Bosch M, Liu T-F, Fordham S, Yuan D, Omary MA, Haranczyk M, Smit B, Zhou H-C (2014) Kinetically tuned dimensional augmentation as a versatile synthetic route towards robust metal–organic frameworks. Nat Commun 5:5723. https://doi.org/10.1038/ncomms6723
Liu Q, Cong H, Deng H (2016) Deciphering the spatial arrangement of metals and correlation to reactivity in multivariate metal–organic frameworks. J Am Chem Soc 138(42):13822–13825. https://doi.org/10.1021/jacs.6b08724
Furukawa H, Gándara F, Zhang Y-B, Jiang J, Queen WL, Hudson MR, Yaghi OM (2014) Water adsorption in porous metal–organic frameworks and related materials. J Am Chem Soc 136(11):4369–4381. https://doi.org/10.1021/ja500330a
Jiang J, Gándara F, Zhang Y-B, Na K, Yaghi OM, Klemperer WG (2014) Superacidity in sulfated metal–organic framework-808. J Am Chem Soc 136(37):12844–12847. https://doi.org/10.1021/ja507119n
Feng D, Wang K, Su J, Liu T-F, Park J, Wei Z, Bosch M, Yakovenko A, Zou X, Zhou H-C (2014) A highly stable zeotype mesoporous zirconium metal–organic framework with ultralarge pores. Angew Chem Int Ed 54(1):149–154. https://doi.org/10.1002/anie.201409334
Wang R, Wang Z, Xu Y, Dai F, Zhang L, Sun D (2014) Porous zirconium metal–organic framework constructed from 2D → 3D interpenetration based on a 3,6-connected kgd net. Inorg Chem 53(14):7086–7088. https://doi.org/10.1021/ic5012764
Feng D, Chung W-C, Wei Z, Gu Z-Y, Jiang H-L, Chen Y-P, Darensbourg DJ, Zhou H-C (2013) Construction of ultrastable porphyrin Zr metal–organic frameworks through linker elimination. J Am Chem Soc 135(45):17105–17110. https://doi.org/10.1021/ja408084j
Alezi D, Spanopoulos I, Tsangarakis C, Shkurenko A, Adil K, Belmabkhout Y, O’Keeffe M, Eddaoudi M, Belmabkhout Y, Trikalitis PN (2016) Reticular chemistry at its best: directed assembly of hexagonal building units into the awaited metal–organic framework with the intricate polybenzene topology, pbz-MOF. J Am Chem Soc 138(39):12767–12770. https://doi.org/10.1021/jacs.6b08176
Yuan S, Lu W, Chen Y-P, Zhang Q, Liu T-F, Feng D, Wang X, Qin J, Zhou H-C (2015) Sequential linker installation: precise placement of functional groups in multivariate metal–organic frameworks. J Am Chem Soc 137(9):3177–3180. https://doi.org/10.1021/ja512762r
Guillerm V, Gross S, Serre C, Devic T, Bauer M, Férey G (2010) A zirconium methacrylate oxocluster as precursor for the low-temperature synthesis of porous zirconium(IV) dicarboxylates. Chem Commun 46(5):767. https://doi.org/10.1039/b914919h
Guillerm V, Grancha T, Imaz I, Juanhuix J, Maspoch D (2018) Zigzag ligands for transversal design in reticular chemistry: unveiling new structural opportunities for metal–organic frameworks. J Am Chem Soc 140(32):10153–10157. https://doi.org/10.1021/jacs.8b07050
Tan Y-X, He Y-P, Zhang J (2011) Pore partition effect on gas sorption properties of an anionic metal–organic framework with exposed Cu2+ coordination sites. Chem Commun 47(38):10647–10649
Liu T-F, Vermeulen NA, Howarth AJ, Li P, Sarjeant AA, Hupp JT, Farha OK (2016) Adding to the arsenal of zirconium-based metal–organic frameworks: the topology as a platform for solvent-assisted metal incorporation. Eur J Inorg Chem 27:4349–4352. https://doi.org/10.1002/ejic.201600627
Morris W, Volosskiy B, Demir S, Gándara F, McGrier PL, Furukawa H, Cascio D, Stoddart JF, Yaghi OM (2012) Synthesis, structure, and metalation of two new highly porous zirconium metal–organic frameworks. Inorg Chem 51(12):6443–6445. https://doi.org/10.1021/ic300825s
Feng D, Gu Z-Y, Li J-R, Jiang H-L, Wei Z, Zhou H-C (2012) Zirconium-metalloporphyrin PCN-222: mesoporous metal–organic frameworks with ultrahigh stability as biomimetic catalysts. Angew Chem Int Ed 51(41):10307–10310. https://doi.org/10.1002/anie.201204475
Mondloch JE, Bury W, Fairen-Jimenez D, Kwon S, DeMarco EJ, Weston MH, Sarjeant AA, Nguyen ST, Stair PC, Snurr RQ, Farha OK, Hupp JT (2013) Vapor-phase metalation by atomic layer deposition in a metal–organic framework. J Am Chem Soc 135(28):10294–10297. https://doi.org/10.1021/ja4050828
Deria P, Mondloch JE, Tylianakis E, Ghosh P, Bury W, Snurr RQ, Hupp JT, Farha OK (2013) Perfluoroalkane functionalization of NU-1000 via solvent-assisted ligand incorporation: synthesis and CO2 adsorption studies. J Am Chem Soc 135(45):16801–16804. https://doi.org/10.1021/ja408959g
Liu J, Ye J, Li Z, Otake K-i, Liao Y, Peters AW, Noh H, Truhlar DG, Gagliardi L, Cramer CJ, Farha OK, Hupp JT (2018) Beyond the active site: tuning the activity and selectivity of a metal–organic framework-supported Ni catalyst for ethylene dimerization. J Am Chem Soc 140(36):11174–11178. https://doi.org/10.1021/jacs.8b06006
Jiang H-L, Feng D, Wang K, Gu Z-Y, Wei Z, Chen Y-P, Zhou H-C (2013) An exceptionally stable, porphyrinic Zr metal–organic framework exhibiting pH-dependent fluorescence. J Am Chem Soc 135(37):13934–13938. https://doi.org/10.1021/ja406844r
Zhang M, Chen Y-P, Bosch M, Gentle T, Wang K, Feng D, Wang ZU, Zhou H-C (2013) Symmetry-guided synthesis of highly porous metal–organic frameworks with fluorite topology. Angew Chem Int Ed 53(3):815–818. https://doi.org/10.1002/anie.201307340
Pang J, Yuan S, Qin J, Liu C, Lollar C, Wu M, Yuan D, Zhou H-C, Hong M (2017) Control the structure of Zr-tetracarboxylate frameworks through steric tuning. J Am Chem Soc 139(46):16939–16945. https://doi.org/10.1021/jacs.7b09973
Wang B, Yang Q, Guo C, Sun Y, Xie L-H, Li J-R (2017) Stable Zr(IV)-based metal–organic frameworks with predesigned functionalized ligands for highly selective detection of Fe(III) ions in water. ACS Appl Mater Interfaces 9(11):10286–10295. https://doi.org/10.1021/acsami.7b00918
Carter JH, Han X, Moreau FY, da Silva I, Nevin A, Godfrey HGW, Tang CC, Yang S, Schröder M (2018) Exceptional adsorption and binding of sulfur dioxide in a robust zirconium-based metal–organic framework. J Am Chem Soc. https://doi.org/10.1021/jacs.8b08433
Pang J, Yuan S, Du D, Lollar C, Zhang L, Wu M, Yuan D, Zhou H-C, Hong M (2017) Flexible zirconium MOFs as bromine-nanocontainers for bromination reactions under ambient conditions. Angew Chem Int Ed 56(46):14622–14626. https://doi.org/10.1002/anie.201709186
Allen F (2002) The Cambridge Structural Database: a quarter of a million crystal structures and rising. Acta Crystallogr Sect B 58(3 Part 1):380–388
Trickett CA, Gagnon KJ, Lee S, Gándara F, Bürgi H-B, Yaghi OM (2015) Definitive molecular level characterization of defects in UiO-66 crystals. Angew Chem Int Ed 54(38):11162–11167. https://doi.org/10.1002/anie.201505461
Kim H, Yang S, Rao SR, Narayanan S, Kapustin EA, Furukawa H, Umans AS, Yaghi OM, Wang EN (2017) Water harvesting from air with metal–organic frameworks powered by natural sunlight. Science 356:430–434
Wißmann G, Schaate A, Lilienthal S, Bremer I, Schneider AM, Behrens P (2012) Modulated synthesis of Zr-fumarate MOF. Microporous Mesoporous Mater 152:64–70. https://doi.org/10.1016/j.micromeso.2011.12.010
Xue D-X, Cairns AJ, Belmabkhout Y, Wojtas L, Liu Y, Alkordi MH, Eddaoudi M (2013) Tunable rare-earth fcu-MOFs: a platform for systematic enhancement of CO2 adsorption energetics and uptake. J Am Chem Soc 135(20):7660–7667. https://doi.org/10.1021/ja401429x
Schoedel A, Li M, Li D, O’Keeffe M, Yaghi OM (2016) Structures of metal–organic frameworks with rod secondary building units. Chem Rev 116(19):12466–12535. https://doi.org/10.1021/acs.chemrev.6b00346
Liu T-F, Feng D, Chen Y-P, Zou L, Bosch M, Yuan S, Wei Z, Fordham S, Wang K, Zhou H-C (2015) Topology-guided design and syntheses of highly stable mesoporous porphyrinic zirconium metal–organic frameworks with high surface area. J Am Chem Soc 137(1):413–419. https://doi.org/10.1021/ja5111317
Wang TC, Bury W, Gómez-Gualdrón DA, Vermeulen NA, Mondloch JE, Deria P, Zhang K, Moghadam PZ, Sarjeant AA, Snurr RQ, Stoddart JF, Hupp JT, Farha OK (2015) Ultrahigh surface area zirconium mofs and insights into the applicability of the BET theory. J Am Chem Soc 137(10):3585–3591. https://doi.org/10.1021/ja512973b
Luebke R, Belmabkhout Y, Weseliński ŁJ, Cairns AJ, Alkordi M, Norton G, Wojtas Ł, Adil K, Eddaoudi M (2015) Versatile rare earth hexanuclear clusters for the design and synthesis of highly-connected ftw-MOFs. Chem Sci 6(7):4095–4102. https://doi.org/10.1039/c5sc00614g
Jiang H, Jia J, Shkurenko A, Chen Z, Adil K, Belmabkhout Y, Weselinski LJ, Assen AH, Xue D-X, O’Keeffe M, Eddaoudi M (2018) Enriching the reticular chemistry repertoire: merged nets approach for the rational design of intricate mixed-linker metal–organic framework platforms. J Am Chem Soc 140(28):8858–8867. https://doi.org/10.1021/jacs.8b04745
Alezi D, Peedikakkal AMP, Weseliński ŁJ, Guillerm V, Belmabkhout Y, Cairns AJ, Chen Z, Wojtas Ł, Eddaoudi M (2015) Quest for highly connected metal–organic framework platforms: rare-earth polynuclear clusters versatility meets net topology needs. J Am Chem Soc 137(16):5421–5430. https://doi.org/10.1021/jacs.5b00450
Chen Z, Weseliński ŁJ, Adil K, Belmabkhout Y, Shkurenko A, Jiang H, Bhatt PM, Guillerm V, Dauzon E, Xue D-X, O’Keeffe M, Eddaoudi M (2017) Applying the power of reticular chemistry to finding the missing alb-MOF platform based on the (6,12)-coordinated edge-transitive net. J Am Chem Soc 139(8):3265–3274. https://doi.org/10.1021/jacs.7b00219
AbdulHalim RG, Bhatt PM, Belmabkhout Y, Shkurenko A, Adil K, Barbour LJ, Eddaoudi M (2017) A fine-tuned metal–organic framework for autonomous indoor moisture control. J Am Chem Soc 139(31):10715–10722. https://doi.org/10.1021/jacs.7b04132
Wang H, Dong X, Lin J, Teat SJ, Jensen S, Cure J, Alexandrov EV, Xia Q, Tan K, Wang Q, Olson DH, Proserpio DM, Chabal YJ, Thonhauser T, Sun J, Han Y, Li J (2018) Topologically guided tuning of Zr-MOF pore structures for highly selective separation of C6 alkane isomers. Nat Commun 9(1):1745. https://doi.org/10.1038/s41467-018-04152-5
Ahnfeldt T, Guillou N, Gunzelmann D, Margiolaki I, Loiseau T, Férey G, Senker J, Stock N (2009) [Al4(OH)2(OCH3)4(H2N-bdc)3]·xH2O: a 12-connected porous metal–organic framework with an unprecedented aluminum-containing brick. Angew Chem Int Ed 48(28):5163–5166. https://doi.org/10.1002/anie.200901409
Dan-Hardi M, Serre C, Frot T, Rozes L, Maurin G, Sanchez C, Férey G (2009) A new photoactive crystalline highly porous titanium(IV) dicarboxylate. J Am Chem Soc 131(31):10857–10859. https://doi.org/10.1021/ja903726m
Zlotea C, Phanon D, Mazaj M, Heurtaux D, Guillerm V, Serre C, Horcajada P, Devic T, Magnier E, Cuevas F, Ferey G, Llewellyn PL, Latroche M (2011) Effect of NH2 and CF3 functionalization on the hydrogen sorption properties of MOFs. Dalton Trans 40(18):4879–4881. https://doi.org/10.1039/C1DT10115C
Fu Y, Sun D, Chen Y, Huang R, Ding Z, Fu X, Li Z (2012) An amine-functionalized titanium metal–organic framework photocatalyst with visible-light-induced activity for CO2 reduction. Angew Chem Int Ed 51(14):3364–3367. https://doi.org/10.1002/anie.201108357
Gándara F, Furukawa H, Lee S, Yaghi OM (2014) High methane storage capacity in aluminum metal–organic frameworks. J Am Chem Soc 136(14):5271–5274. https://doi.org/10.1021/ja501606h
Sumida K, Hill MR, Horike S, Dailly A, Long JR (2009) Synthesis and hydrogen storage properties of Be12(OH)12(1,3,5-benzenetribenzoate)4. J Am Chem Soc 131(42):15120–15121. https://doi.org/10.1021/ja9072707
Lee S, Kapustin EA, Yaghi OM (2016) Coordinative alignment of molecules in chiral metal–organic frameworks. Science 353(6301):808. https://doi.org/10.1126/science.aaf9135
Kapustin EA, Lee S, Alshammari AS, Yaghi OM (2017) Molecular retrofitting adapts a metal–organic framework to extreme pressure. ACS Cent Sci 3(6):662–667. https://doi.org/10.1021/acscentsci.7b00169
Guillerm V, Weseliński Łukasz J, Belmabkhout Y, Cairns AJ, D’Elia V, WojtasŁukasz, Adil K, Eddaoudi M (2014) Discovery and introduction of a (3,18)-connected net as an ideal blueprint for the design of metal–organic frameworks. Nat Chem 6(8):673–680. https://doi.org/10.1038/nchem.1982. http://www.nature.com/nchem/journal/v6/n8/abs/nchem.1982.html#supplementary-information
Yuan S, Chen Y-P, Qin J-S, Lu W, Zou L, Zhang Q, Wang X, Sun X, Zhou H-C (2016) Linker installation: engineering pore environment with precisely placed functionalities in zirconium MOFs. J Am Chem Soc 138(28):8912–8919. https://doi.org/10.1021/jacs.6b04501
Yuan S, Zou L, Li H, Chen Y-P, Qin J, Zhang Q, Lu W, Hall MB, Zhou H-C (2016) Flexible zirconium metal–organic frameworks as bioinspired switchable catalysts. Angew Chem Int Ed 55(36):10776–10780. https://doi.org/10.1002/anie.201604313
Pang J, Yuan S, Qin J, Wu M, Lollar CT, Li J, Huang N, Li B, Zhang P, Zhou H-C (2018) Enhancing pore-environment complexity using a trapezoidal linker: toward stepwise assembly of multivariate quinary metal–organic frameworks. J Am Chem Soc 140(39):12328–12332. https://doi.org/10.1021/jacs.8b07411
Zhang X, Frey BL, Chen Y-S, Zhang J (2018) Topology-guided stepwise insertion of three secondary linkers in zirconium metal–organic frameworks. J Am Chem Soc 140(24):7710–7715. https://doi.org/10.1021/jacs.8b04277
Acknowledgements
Funding of MOF research in the Schoedel group is supported by startup funds from the Florida Institute of Technology, United States. S. R. gratefully acknowledges financial assistance from University of Hail, Hail, Kingdom of Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection “Metal–Organic Framework: From Design to Applications”, edited by Xian-He Bu, Michael J. Zaworotko, and Zhenjie Zhang.
Rights and permissions
About this article
Cite this article
Schoedel, A., Rajeh, S. Why Design Matters: From Decorated Metal Oxide Clusters to Functional Metal–Organic Frameworks. Top Curr Chem (Z) 378, 19 (2020). https://doi.org/10.1007/s41061-020-0281-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-020-0281-0