Abstract
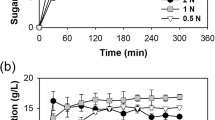
The third-generation bioethanol production depends on the utilization of algae as raw material and an excellent source of polysaccharides, which can be fermented to bioethanol. The microalga Chlorella vulgaris is suitable for bioethanol production due to its carbohydrate content. In this study, the chemical pretreatments of acidic and alkaline pretreatments of Chlorella vulgaris biomass showed that the maximum reducing sugars and total carbohydrate concentrations produced from acidic pretreatment of the algal biomass by using 2% sulfuric acid (H2SO4) at 120 °C for 30 min were (24.77 g/100 g dried biomass) and (16.08 g/100 g dried biomass), respectively. The bioethanol released from the biomass was increased gradually until the 5th day (14.32 g/100 g dried biomass). The maximum bioethanol produced after 2% (w/v) of sodium hydroxide (NaOH) at 120 °C for 30 min. alkaline pretreatment was (21.54 g/100 g dried biomass) reducing sugar, (15.72 g/100 g dried biomass) total carbohydrate and the produced bioethanol concentration on the 4th day (7.74 g/100 g dried biomass). At the same time, the physical pretreatments were done by microwaves and ultrasonication. The highest reducing sugars after ultrasonication, (18.97 g/100 g dried biomass) which fermented to (16.19 g/100 g dried biomass) bioethanol at the 4th fermentation day. While microwaves pretreatment reached (11.22 g/ 100 g dried biomass) reducing sugars, that synthesis (2.91 g/100 g dried biomass) at the 5th fermentation day. This work concluded that 2% H2SO4 was the best treatment method, which resulted in the highest concentrations of reducing sugars, total carbohydrate, and bioethanol.
Graphic Abstract



Similar content being viewed by others
References
Abomohra A, Elasyed M, Sakkimuthu S, El-Sheekh M, Hanel D (2020) Potential of fat, oil and grease (FOG) for biodiesel production: a critical review on the recent progress and future perspectives. Prog Energy Combust Sci 81:100868
Akhtara N, Karnwala A, Upadhyaya AK, Paulb S, Mannan MA (2018) Saccharomyces cerevisiae bio-ethanol production, a sustainable energy alternative. Asian J Microbiol Biotechnol Environ Sci 20:202–206
Andrés F, Barajas S, Angel DGD, Viatcheslav K (2014) Effect of thermal pretreatment on fermentable sugar production of Chlorella Vulgaris. Chem Eng Trans 37:655–660
Balat M, Balat H, Öz C (2008) Progress in bioethanol processing. Prog Energy Combust Sci 34(5):551–573
Caputi A, Veda M, Brown T (1968) Spectrophotometric determination of ethanol in wine. Am J Enol Vitic 19(3):160–165
Cen WH, Huang MY, Chang JS, Chen CY (2014) Thermal decomposition dynamics and severity of microalgae residues in torrefaction. Bioresour Technol 169:258–264
Cheng J, Sun J, Huang Y, Feng J, Zhou J, Cen K (2013) Dynamic microstructures and fractal characterization of cell wall disruption for microwave irradiation-assisted lipid extraction from wet microalgae. Bioresour Technol 150:67–72
Chikako A, Keita D, Chizuru S, Yoshitoshi N (2012) Efficient extraction of starch from microalgae using ultrasonic homogenizer and its conversion into ethanol by simultaneous saccharification and fermentation. Sci Res Publ 3(4):175–179
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
El-Sheekh MM, Hamouda RA (2016) Lipids extraction from the green alga Ankistrodesmus falcatus using different methods. Rendiconti Lincei 27(3):589–595
El-Sheekh MM, Ismail A-MS, El-Abd MA, Hegazy EM, El-Diwany AI (2009) Effective technological pectinases by Aspergillus carneus NRC1 utilizing the Egyptian orange juice industry scraps. Int Biodeterior Biodegrad 63(1):12–18
El-Sheekh MM, Bedaiwy MY, Osman MEH, Ismail M (2014) Influence of molasses on growth, biochemical composition and ethanol production of the green algae Chlorella vulgaris and Scenedesmus obliquus. J Agric Eng Biotechnol 2(2):20–28
Eshaq FS, Ali MN, Mohd MK (2010) Production of bioethanol from next generation feed-stock alga Spirogyra species. Int J Eng Sci Technol 3(2):1749–1755
Fan LT, Gharpuray MM, Lee YH (1987) Cellulose hydrolysis. Biotechnol Monogr 3:21–119
Girio FM, Fonseca C, Carvalheiro F, Duarte L, Marques S, Bogel-Łukasik R (2010) Hemicelluloses for fuel ethanol: are view. Bioresour Technol 101(13):4775–4800
Harun R, Jason WSY, Cherrington T, Danquah MK (2011) Exploring alkaline pretreatment of microalgal biomass for bioethanol production. Appl Energy 88(10):3464–3467
Hassan LHS, El-Hefnawy MAA, Morsy EM, Abou-El-Souod GW (2016) Production of Bioethanol from microalgae. MSc. Thesis, University of Menoufia, Egypt, pp 35–36
Hendriks ATWM, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100(1):10–18
Ho SH, Huang SW, Chen CY, Hasunuma T, Kondo A, Chang JS (2013) Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour Technol 135:191–198
Ismail MM, Ismail GA, El-Sheekh MM (2020) Potential assessment of some micro- and macroalgal species for bioethanol and biodiesel production. Energy Sources Part A Recovery Util Environ Effects. https://doi.org/10.1080/15567036.2020.1758853
Jeon BH, Choi JA, Kimm HC, Hwangm JH (2013) Ultrasonic disintegration of microalgal biomass and consequent improvement of bioaccessibility/bioavailability in microbial fermentation. Biotechnol Biofuels 6(1):37
John RP, Anisha GS, Nampoothiri KM, Pandey A (2011) Micro and macroalgal biomass: a renewable source for bioethanol. Biores Technol 102(1):186–193
Keshwani DR, Cheng JJ, Burns JC, Li L, Chiang V (2007) Microwave pretreatment of switchgrass to enhance enzymatic hydrolysis. In: Conference presentations and white papers: biological systems engineering. Paper 35. University of Nebraska, Lincoln. American Society of Agricultural and Biological Engineers
Koullas DP, Christakopoulos P, Kekos D, Macris BJ, Koukios EG (1992) Correlating the effect of pretreatment on the enzymatic hydrolysis of straw. Biotechnol Bioeng 39(1):113–116
Kuakpetoon D, Wang YJ (2007) Internal structure and physicochemical properties of corn starches as revealed by chemical surface gelatinization. Carbohydr Res 342(15):2253–2263
Larsson S, Palmqvist E, Hahn-Hägerdal B, Tengborg C, Stenberg K, Zacchi G, Nilvebrant NO (1999) The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb Technol 24(3):151–159
Lastella G, Testa C, Cornacchia G, Notornicola M, Voltasio F, Sharma VK (2002) Anaerobic digestion of semi-solid organic waste: biogas production and its purification. Energy Convers Manag 43(1):63–75
Lee YY, Iyer P, Torget RW (1999) Dilute-acid hydrolysis of lignocellulosic biomass. Adv Biochem Eng Biotechnol 65:93–115
Lee S, Oh Y, Kim D, Kwon D, Lee C, Lee J (2011) Converting carbohydrates extracted from marine algae into ethanol using various ethanolic Escherichia coli strains. Appl Biochem Biotechnol 164(6):878–888
Lenihan P, Orozco A, O’Neill E, Ahmad MNM, Rooney DW, Walker GM (2010) Dilute acid hydrolysis of lignocellulosic biomass. Chem Eng J 156(2):395–403
Li K, Liu S, Liu X (2014) An overview of algae bioethanol production. Int J Energy Res 38(8):965–977
Martin C, Klinke HB, Thomsen AB (2007) Wet oxidation as a pretreatment method for enhancing the enzymatic convertibility of sugarcane bagasse. Enzyme Microb Technol 40(3):426–432
Miller DL (1959) Use of dinitrosaliylic acid as reagent of reducing sugars. Anal Chem 31(3):426–428
Miranda JR, Passarinho PC, Gouveia L (2012a) Pretreatment optimization of Scenedesmus obliquus microalga for bioethanol production. Bioresour Technol 104:342–348
Miranda JR, Passarinho PC, Gouveia L (2012b) Pretreatment optimization of Scenedesmus obliquus microalga for bioethanol production. Biores Technol 104:342–348
Nigam PS, Singh A (2010) Production of liquid biofuels from renewable resources. Prog Energy Combust Sci, 37(1):52–68
Ometto F, Quiroga G, Pšenička P, Whitton R, Jefferson B, Villa R (2014) Impacts of microalgae pretreatments for improved anaerobic digestion: thermal treatment, thermal hydrolysis, ultrasound and enzymatic hydrolysis. Water Res 65:350–361
Park JH, Hong JY, Jang HC, Oh SG, Kim SH, Yoon JJ, Kim YJ (2012) Use of Gelidium amansii as a promising resource for bioethanol: a practical approach for continuous dilute-acid hydrolysis and fermentation. Bioresour Technol 108:83–88
Ratledge C, Cohen Z (2008) Microbial and algal oils: do they have a future for biodiesel or as commodity oils. Lipid Technol 20(7):155–160
Sayadi MH, Ghatnekar SD, Kavian MF (2011) Algae a promising alternative for biofuel. Proc Int Acad Ecol Environ Sci 1(2):112–124
Tabil L, Adapa P, Kashaninejad M (2011) Biomass feedstock preprocessing—part 1: pretreatment. In: Santos MAB (ed) Biofuels engineering process technology. In Tech, Rejika, pp 411–438
Talebnia F, Karakashev D, Angelidaki I (2009) Production of bioethanol from wheat straw: an overview on pretreatment, hydrolysis, and fermentation. Bioresour Technol 101(13):4744–4753
Vincent M, Pometto AL, van Leeuwen J (2014) Ethanol production via simultaneous saccharification and fermentation of sodium hydroxide treated corn stover using Phanerochaete chrysosporium and Gloeophyllum trabeum. Bioresour Technol 158:1–6
Wan C, Zhou Y, Li Y (2011) Liquid hot water and alkaline pretreatment of soybean straw for improving cellulose digestibility. Bioresour Technol 102(10):6254–6259
Wang Z, Keshwani DR, Redding AP, Cheng JJ (2010) Sodium hydroxide pretreatment and enzymatic hydrolysis of coastal Bermuda grass. Bioresour Technol 101(10):3583–3585
Wang Z, Li R, Xu J, Marita JM, Hatfield RD, Qu R, Cheng JJ (2012) Sodium hydroxide pretreatment of genetically modified switchgrass for improved enzymatic release of sugars. Bioresour Technol 110:364–370
Wang YZ, Liao QL, Zhu X, Ran Y, Hou C-J (2015) Solid simultaneous saccharification and fermentation of rice straw for bioethanol production using nitrogen gas stripping. R Soc Chem Adv 5(68):55328–55335
Wongjewboot I, Kangsadan T, Kongruang S (2010) Ethanol production from rice straw using ultrasonic pretreatment. In: International conference on chemistry and chemical engineering, Kyoto, Japan. 16–193
Zhao G, Chen X, Wang L, Zhou S, Feng H, Chen WN, Lau R (2013) Ultrasound assisted extraction of carbohydrates from microalgae as feedstock for yeast fermentation. Bioresour Technol 128:337–344
Zheng Y, Pan Z, Hang R (2009) Overview of biomass pretreatment for cellulosic ethanol production. Int J Agric Biol Eng 2(3):51–68
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Abou El-Souod, G.W., Morsy, E.M., Hassan, L.H.S. et al. Efficient Saccharification of the Microalga Chlorella vulgaris and its Conversion into Ethanol by Fermentation. Iran J Sci Technol Trans Sci 45, 767–774 (2021). https://doi.org/10.1007/s40995-021-01097-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-021-01097-1