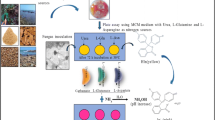
Abstract
Microbial l-asparaginases have received significant attention due to their potential for applications in cancer treatment and for preparation of acrylamide-free baked and fried food. Eleven fungal-type strains were obtained from Iranian Biological Resource and screened for production of l-asparaginase using dye-based plate assay on modified Czapek Dox medium. The efficiency of three different dye indicators including phenol red, bromothymol blue and cresol red was evaluated. Production mode was also evaluated by the agar well diffusion method. A comparative study was carried out to evaluate l-asparaginase production in submerged condition by enzymatic assay using Nessler’s reagent. Optimization of the enzyme production by the selected superior strain was performed using one factor at a time method for temperature, inoculum and different carbon and nitrogen sources. Five-type strains showed positive l-asparaginase activity in preliminary screening. Phenol red and bromothymol blue showed better differentiable zone of hydrolysis and consistent results in comparison with cresol red. The results also indicated that l-asparaginase production mode is extracellular. l-asparaginase production by Fereydounia khargensis IBRC-M 30116T which showed the highest zone of color change was then optimized. The optimum enzyme activity of 61.3 U. mL−1 was obtained with 9% inoculum, at a temperature of 30 °C and at the presence of ammonium chloride as nitrogen source and with glycerol as the carbon sources. The study revealed that, apart from bacteria, yeast and yeast-like microorganisms could be considered as novel and appropriate sources for production of the high yield of l-asparaginase.


Similar content being viewed by others
Availability of Data and Material
The data that support the finding of this study are openly available in NCBI.
References
Alimadadi N, Soudi MR, Wang SA, Wang QM, Talebpour Z, Bai FY (2016) Starmerella orientalis f.a., sp. nov., an ascomycetous yeast species isolated from flowers. IJSEM 66(3):1476–1481. https://doi.org/10.1099/ijsem.0.000905
Ashok A, Doriya K, Rao JV, Qureshi A, Tiwari AK, Kumar DS (2019) Microbes producing l-asparaginase free of glutaminase and urease isolated from extreme locations of antarctic soil and moss. Sci Rep 9:1423. https://doi.org/10.1038/s41598-018-38094-1
Batool T, Makky EA, Jalal M, Yusoff MM (2016) A comprehensive review on l-asparaginase and its applications. Appl Biochem Biotechnol 178(5):900–923. https://doi.org/10.1007/s12010-015-1917-3
Brumano LP, Santos da Silva FV, Costa-Silva TL, Apolinário AC, Madalena Santos JHP, Kleingesinds EK, Monteiro G, Rangel-Yagui CO, Benyahia B, Junior AP (2019) Development of l-asparaginase biobetters: current research status and review of the desirable quality profiles. Front Bioeng Biotechnol 6:212. https://doi.org/10.3389/fbioe.2018.00212
Cachumba JJ, Antunes FA, Peres GF, Brumano LP, Santos JC, Da Silva SS (2016) Current applications and different approaches for microbial l-asparaginase production. Braz J Microbiol 1(1):77–85. https://doi.org/10.1016/j.bjm.2016.10.004
Chand S, Mahajan RV, Prasad JP, Sahoo DK, Mihooliya KN, Dhar MS, Sharma G (2020) A comprehensive review on microbial l-asparaginase: bioprocessing, characterization and industrial applications. Biotechnol Appl Biochem. https://doi.org/10.1002/bab.1888
Doriya K, Kumar DS (2016) Isolation and screening of L-asparaginase free of glutaminase and urease from fungal sp. 3 Biotech 6:239. https://doi.org/10.1007/s13205-016-0544-1
El-Hadi AA, El-Refai HA, Shafei MS, Zaki R (2017) Statistical optimization of l-asparaginase production by using Fusarium solani. Egypt Pharmaceut J 16(1):16–23. https://doi.org/10.4103/1687-4315.205825
El-Hadi AA, Ahmed HM, Hamzawy RA (2019) Optimization and characterization of L-asparaginase production by a novel isolated Streptomyces spp. strain. Egypt Pharmaceut J 18(2):111–122. https://doi.org/10.4103/epj.epj_23_18
El-Naggar NA, Deraz SF, El-Ewasy SM, Suddek GM (2018) Purification, characterization and immunogenicity assessment of glutaminase free l-asparaginase from Streptomyces brollosae NEAE-115. BMC Pharmacol Toxicol 19(1):51. https://doi.org/10.1186/s40360-018-0242-1
Ghosh S, Murthy S, Govindasamy S, Chandrasekaran M (2013) Optimization of l-asparaginase production by Serratia marcescens (NCIM 2919) under solid state fermentation using coconut oil cake. Sustain Chem Process 1:9. https://doi.org/10.1186/2043-7129-1-9
Imada A, Igarasi S, Nakahama K, Isono M (1973) Asparaginase and glutaminase activities of micro-organisms. J Gen Microbiol 76(1):85–99. https://doi.org/10.1099/00221287-76-1-85
Krishnapura PR, Belur PB, Subramanya S (2016) A critical review on properties and applications of microbial l-asparaginases. Crit Rev Microbiol 42(5):720–737. https://doi.org/10.3109/1040841X.2015.1022505
Mahajan RV, Saran S, Saxena RK, Srivastava AK (2013) A rapid, efficient and sensitive plate assay for detection and screening of l-asparaginase-producing microorganisms. FEMS Microbiol Lett 341(2):122–126. https://doi.org/10.1111/1574-6968.12100
Mihooliya KN, Nandal J, Swami L, Verma H, Chopra L (2017) A new pH indicator dye-based method for rapid and efficient screening of l-asparaginase producing microorganisms. Enzyme Microb Technol 107:72–81. https://doi.org/10.1016/j.enzmictec.2017.08.004
Moguel IS (2018) Production of l-asparaginase of pharmaceutical interest from yeasts isolated from the Antarctic continent. Dissertation, University of Sao Paulo, Faculty of Pharmacetical Sciences
Nandal P, Ravella SR, Kuhad RC (2013) Laccase production by Coriolopsis caperata RCK2011: optimization under solid state fermentation by Taguchi DOE methodology. Sci Rep 3:1386. https://doi.org/10.1038/srep01386
Nasr S, Soudi MR, Zamanzadeh Nasrabadi SM, Moshtaghi Nikou M, Salmanian AH, Nguyen HDT (2014a) Basidioascus persicus: a new yeast-like species of the Geminibasidiales isolated from Iran soil. IJSEM 64:3046–3052. https://doi.org/10.1099/ijs.0.062380-0
Nasr S, Soudi MR, Shahzadeh Fazeli SA, Nguyen HDT, Lutz M, Piątek M (2014b) Expanding evolutionary diversity in the Ustilaginomycotina: fereydouniaceae fam. nov. and Fereydounia gen. nov., the first urocystidalean yeast lineage. Mycol Prog 13:1012. https://doi.org/10.1007/s11557-014-1012-0
Nasr S, Nguyen HDT, Soudi MR, Shahzadeh Fazeli SA, Sipiczki M (2016) Wickerhamomyces orientalis f. a., sp. nov.:anascomycetous yeast species belonging to the Wickerhamomyces clade. IJSEM 66(7):2534–2539. https://doi.org/10.1099/ijsem.0.001096
Nasr S, Mohammadimehr M, Geranpayeh Vaghei M, Amoozegar MA, Shahzadeh Fazeli SA, Yurkov A (2017) Jaminaea pallidilutea sp. nov. (Microstromatales), a basidiomycetous yeast isolated from plant material of mangrove forests in Iran. IJSEM 67(11):4405–4408. https://doi.org/10.1099/ijsem.0.002302
Nasr S, Mohammadimehr M, Geranpayeh Vaghei M, Amoozegar MA, Shahzadeh Fazeli SA (2018a) Aureobasidium mangrovei sp. nov., an ascomycetous species recovered from Hara protected forests in Persian Gulf, Iran. Antonie Van Leeuwenhoek 111(9):1697–1705. https://doi.org/10.1007/s10482-018-1059-z
Nasr S, Bien S, Soudi MR, Alimadadi N, Shahzadeh Fazeli SA, Damm U (2018b) Novel Collophorina and Coniochaeta species from Euphorbia polycaulis, an endemic plant in Iran. Mycol Prog 17:755–771. https://doi.org/10.1007/s11557-018-1382-9
Nasr S, Lutz M, Amoozegar MA, Eparvier V, Stien D, Shahzadeh Fazeli SA, Yurkov A (2018c) Graphiola fimbriata: the first species of Graphiolaceae (Exobasidiales, Basidiomycota) described only based on its yeast stage. Mycol Prog 18:359–368. https://doi.org/10.1007/s11557-018-1450-1
Nouri H, Moghimi H, Geranpayeh Vaghei M, Nasr S (2017) Blastobotrys persicus sp. nov., an ascomycetous yeast species isolated from cave soil. Antonie Van Leeuwenhoek 111:517–524. https://doi.org/10.1007/s10482-017-0972-x
Qeshmi FI, Homaei A, Fernandes P, Javadpour S (2018) Marine microbial l-asparaginase: biochemistry, molecular approaches and applications in tumor therapy and in food industry. Microbiol Res 208:99–112. https://doi.org/10.1016/j.micres.2018.01.011
Rani SA, Sundaram L, Vasantha PB (2012) Isolation and screening of l-asparaginase producing fungi from soil samples. Int J Pharm Pharm Sci 4(1):279–282
Talluri VP, Lanka SS, Saladi VR (2019) Statistical optimization of process parameters by central composite design (CCD) for an enhanced production of l-asparaginase by Myroides gitamensis BSH-3, a novel species. Avicenna J Med Biotechnol 11(1):59–66
Thirunavukkarasu N, Suryanarayanan TS, Murali TS, Ravishankar JP, Gummadi SN (2011) l-asparaginase from marine derived fungal endophytes of seaweeds. Mycosphere 2:147–155
Trivedi R, Padalia U (2016) l-Asparaginase as an anti-carcinogenic agent. IJESC 6:2824–2826
Vaishali P, Bhupendra NT (2017) A rapid and efficient dye based plate assay technique for screening of l-asparaginase producing fungal strains. J. Microb Biochem Technol 9(4):162–168. https://doi.org/10.4172/1948-5948.1000361
Vala KA, Dave BP (2016) l-Asparaginases from fungi: a mini review. J Bacteriol Mycol 3(1):181–182
Venil CK, Lakshmanaperumalsamy P (2008) Solid state fermentation for production of l-asparaginase in rice bran by Serratia marcescens SB08. Int J Microbiol 7:30. https://doi.org/10.5580/1d06
Vimal A, Kumar A (2017a) Biotechnological production and practical application of L asparaginase enzyme. Biotechnol Genet Eng Rev 33(1):40–61. https://doi.org/10.1080/02648725.2017.1357294
Vimal A, Kumar A (2017b) In vitro screening and in silico validation revealed key microbes for higher production of significant therapeutic enzyme l-asparaginase. Enzyme Microb Technol 98:9–17. https://doi.org/10.1016/j.enzmictec.2016.12.001
Wriston J, Yellin T (1973) l-asparaginase: a review. Adv Enzymol Relat Areas Mol Biol 39:185–248. https://doi.org/10.1002/9780470122846.ch3
Acknowledgements
The authors gratefully acknowledge financial support from the Iranian Biological Resource Centre (IBRC), ACECR.
Funding
This study was funded by Iranian Biological Resource Centre (IBRC), ACECR, Iran.
Author information
Authors and Affiliations
Contributions
All authors have contributed sufficiently in the planning, execution, or analysis of this study to be included as authors.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Consent to Participate
The authors declare that they voluntarily agree to participate in this research study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fazeli, N., Alimadadi, N. & Nasr, S. Screening and Optimization of Process Parameters for the Production of l-asparaginase by Indigenous Fungal-Type Strains. Iran J Sci Technol Trans Sci 45, 409–416 (2021). https://doi.org/10.1007/s40995-020-01056-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-020-01056-2