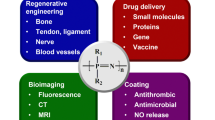
Abstract
Although many traditional organic polymers have been evaluated for uses in biology and medicine, relatively few have proved to be satisfactory for crucial uses such as surgical sutures or mesh, tissue engineering substrates, controlled drug delivery, or soft matter. A reason for this is that most large-volume commercial polymers are optimized for some mechanical engineering purpose, and biomedical compatibility or bioerodibility is not one of the target properties. Thus, biomedical scientists and engineers have been forced to improvise and compromise by using widely available non-biological polymers, mainly because those materials are already available in commercial quantities. Polyphosphazenes offer an opportunity to solve many of these problems.
Lay Summary
A crucial need exists for new polymers that can be utilized in orthopedics, cardiovascular, dental, neural, or drug delivery applications, yet very few long-existing polymers have properties that are ideal for these medical uses. Our research seeks to design and find methods to synthesize polymeric materials that are specifically designed to solve a range of medical challenges. In our program, we make use of an unusual polymer backbone comprised of alternating phosphorus and nitrogen atoms to which are attached a wide range of organic side groups. This system is unique in the wide range of different property combinations that can be generated, and many of these combinations can be matched precisely to the needs of medical materials.






Similar content being viewed by others
References
Allcock HR. Chemistry and applications of polyphosphazenes. Hoboken, N.J: Wiley; 2003.
Allcock HR, Kugel RL. Synthesis of high polymeric alkoxy- and aryloxy- phosphonitriles. J Am Chem Soc. 1965;87:4216–7.
Allcock HR, Kugel RL, Valan KJ. High molecular weight poly(alkoxy- and aryloxyphosphazenes). Inorg Chem. 1966;5:1709–15.
Allcock HR, Kugel RL. High molecular weight poly(diaminophosphazenes). Inorg Chem. 1966;5:1716–8.
Wisian-Neilson P. Polyphosphazenes from condensation polymerization, Ch. 10. In: Andrianov AK, editor. Biomedical Applications of Polyphosphazenes. Hoboken, N. J: Wiley; 2009. p. 155–16.
Matyjaszewski K, Uli F, Montague RA, White ML. Synthesis of polyphosphazenes from phosphoranimines and phosphine azides. Polymer. 1994;35:5005–11.
Honeyman CH, Manners I, Morrissey CT, Allcock HR. Ambient temperature synthesis of poly(dichlorophosphazene) with molecular weight control. J Am Chem Soc. 1995;117:7035–6.
Allcock HR, Nelson JM, Reeves SD, Honeyman CH, Manners I. Ambient temperature direct synthesis of poly(organophosphazenes) via the “living” cationic polymerization of organo-substituted phosphoramines. Macromolecules. 1997;30:50–6.
Nelson JM, Allcock HR. Synthesis of triarmed star polyphosphazenes via the living cationic polymerization of phosphoranimines at ambient temperatures. Macromolecules. 1997;30:1854–6.
Rose SH. Synthesis of phosphonitrilic fluoroelastomers. Polym Lett. 1968;6:837–9.
Tate DP. Polyphosphazene elastomers. J Polymer Sci Polym Lett. 1974;48:33–45.
Penton H, Polyphosphazenes R. Performance polymers for specialty applications. Ch 21. In: Zeldin M, et al., editors. Inorganic and Organometallic Polymers. ACS Symposium Series. Washington, DC: American Chemical Society; 1988.
Xu L, Li Z, Tian Z, Chen C, Allcock HR, Siedlecki C. A new textured polyphosphazene biomaterial with improved blood coagulation and microbial infection responses. Acta Biomater. 2018;67:87–98.
Modzelewski T, Wilts E, Allcock HR. Elastomeric polyphosphazenes with phenoxy-cyclotriphosphazene side groups. 2015;48:7543–9.
Modzelewski T, Wonderling NM, Allcock HR. Phosphazene elastomers containing interdigitated oligo-p-phenoxy side groups. 2015;48:4882–90.
Modzelewski T, Allcock HR. An unusual polymer architecture for the generation of elastomeric properties in fluorinated polyphosphazenes. Macromolecules. 2014;47:6776–82.
Prange R, Allcock HR. Telechelic syntheses of the first phosphazene-siloxane block copolymers. Macromolecules. 1999;32:6390–2.
Allcock HR, Kuharcik SE, Nelson CJ. The synthesis and characterization of amino-organosiloxane-bearing polyphosphazenes: new properties by the elimination of hydrogen bonding. Macromolecules. 1996;29:3686–93.
Allcock HR, Kuharcik SE. Hybrid phosphazene-organosilicon polymers. Part II. High polymer and materials synthesis and properties. J Inorg Organ Polym. 1996;6:1–41.
Allcock HR, Smith DE. Surface studies of poly(organophosphazenes) containing dimethylsiloxane grafts. Chem Mater. 1995;7:1469–74.
Powell ES, Chang Y, Allcock HR, Kim C. Self-organization of amphiphilic polyphosphazene-polystyrene block copolymers. Polymer Preprints (ACS Div. Polymer Chem.). 2004;45:49.
Allcock HR, Powell ES, Chang Y, Kim C. Synthesis and micellar behavior of amphiphilic polystyrene-poly[bis(methoxyethoxyethoxy)phosphazene] co-polymers. Macromolecules. 2004;37:7163–7.
Allcock HR, Powell ES, Maher AE, Berda EB. Poly(methylmethacrylate)- graft-poly[bis(trifluoroethoxy)phosphazene] copolymers; synthesis characterization and effects of polyphosphazene incorporation. Macro- molecules. 2004;37:5824–9.
Laurencin C, Koh HJ, Neenan TX, Allcock HR, Langer RS. Controlled release using a new bioerodible polyphosphazene matrix system. J Biomed Res. 1987;21:1231–46.
Allcock HR, Morozowich NL. Bioerodible polyphosphazenes and their medical potential. Royal Soc Chem. 2012;3:578–90.
Nichol JL, Hotham IT, Allcock HR. Ethoxyphosphazene polymers and their hydrolytic behavior. Macromolecules. 2014;109:92–6.
Allcock HR, Scopelianos AG. Synthesis of sugar-substituted cyclic and polymeric phosphazenes and their oxidation, reduction, and acetylation reactions. Macromolecules. 1983;16:715–9.
Peach MS, Kumbar SG, James R, Toti US, Balasubramaniam D, Deng M, et al. Design and optimization of polyphosphazene functionalized fiber matrices for soft tissue engineering. J Biomed Nano- Tech. 2012;8:107–24.
Deng M, Kumbar SG, Nair LS, Weikel AL, Allcock HR, Laurencin CT. Biomimetic structures: biological implications of dipeptide-substituted phosphazene-polymer blend nanofiber matrices for load bearing bone regeneration. Adv Functional Mater. 2011;21:2641–51.
Ogueri KS, Allcock HR, Laurencin CT. Polyphosphazene polymers (2019). In: Encyclopedia of Polymer Science and Technology (Accepted).
Deng M, Allcock HR, Laurencin CT, Kumbar SG. Polyphosphazenes as biomaterials. In: Dumitriv V, Popa V, editors. Polymeric Biomaterials. 3rd ed. Boca Raton: CRC Press.
Peach MS, Ramos DM, James R, Morozowich NL, Mazzocca AD, Doty SB, et al. Engineered stem cell niche matrices for rotator cuff tendon regenerative engineering. PLOS. 2017:1–19.
Cohen S, Bano MC, Visscher KB, Chow M, Allcock HR, Langer RS. An ionically-crosslinkable polyphosphazene: a novel polymer for micro- encapsulation. J Am Chem Soc. 1990;112:7832–3.
Allcock HR, Powell ES, Chang Y, Kim C. Synthesis and micellar behavior of amphiphilic polystyrene-poly[bis(methoxyethoxyethoxy)phosphazene] block copolymers. Macromolecules. 2004;37:7163–7.
Allcock HR, Allen RW, O’Brien JP. Synthesis of platinum derivatives of polymeric and cyclic phosphazenes. J Am Chem Soc. 1977;99:3984–7.
Jun YJ, Kim JI, Jun MJ, Sohn YS. Selective tumor targeting by enhanced permeability and retention effect. Synthesis and antitumor activity of polyphosphazene platinum (II) conjugates. J Inorg Biochem. 2005;99(8):1593–601.
Allcock HR, Kwon S. An ionically-crosslinkable poly[di(phosphazene: Poly- carboxylatophenoxy phosphazene)]. 1989;22:75–9.
Selin V, Albright V, Ankner JF, Marin A, Andrianov AK, Sukhishvili SA. Biocompatible nanocoatings of fluorinated polyphosphazenes through aqueous assembly. ACS Appl Mater Interfaces. 2018;10(11):9756–64.
Andrianov AK, Martinez AP, Weidman JL, Marin A, Fuerst TR. Biodegradable PEGylated polyelectrolyte nanocomplexes for protein delivery. Biomacromolecules. 2018;19:3467–78.
Allcock HR, Fuller TJ. Phosphazene high polymers with steroidal side groups. Macromolecules. 1980;13:1338–45.
Crommen J, Vandorpe J, Schacht E. Degradable polyphosphazenes for biomedical applications. J. Controlled Release. 1993;24:I67–180.
Schacht, E.; Crommen, J. Bioerodible Sustained Release Implants. U.S. Patent 4975280A (to Ethyl Corp.) 1989.
Hindenlang MD, Soudakov AA, Imler GH, Laurencin CT, Nair LS, Allcock HR. Iodine-containing radio-opaque polyphosphazenes R. S C. Polym Chem. 2010;1:1467–74.
Chhour P, Gallo N, Cheheltani R, Williams D, Al-Zaki A, Paik T, et al. Nanodisco balls: control over surface versus core loading of diagnostically-active nanocrystals into polymer nanoparticles. ACS Nano. 2014;8(9):9143–53.
Bates MC, Yousaf A, Sun L, Barakat M, Kueller A. Translational research and early favorable clinical results of a novel polyphosphazene (polyzene-F) nanocoating. Regen Eng Transl Med. 2019. https://doi.org/10.1007/s40883-019-00097-3.
Acknowledgments
The author acknowledges the contributions to our program by 108 graduate students, numerous undergraduates, 36 postdoctoral scientists, and many collaborators. The biomedical funding at different times has been through the U.S. National Institutes of Health, National Science Foundation, the U.S. Army Research Office, The Pennsylvania State University, and private donations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Allcock, H.R. The Background and Scope of Polyphosphazenes as Biomedical Materials. Regen. Eng. Transl. Med. 7, 66–75 (2021). https://doi.org/10.1007/s40883-019-00128-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-019-00128-z