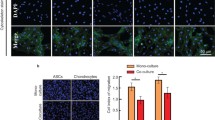
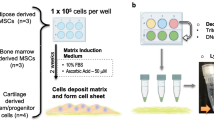
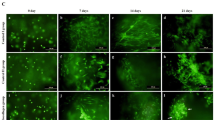
Abstract
Donor cell scarcity is a key barrier for clinical translation of chondrocytes for regenerating cartilage. To overcome this challenge, recent studies have utilized mixed populations of chondrocytes and adult stem cells to show synergistic interactions during 3D co-culture using hydrogels or pellets. Yet, how different co-culture models impact the synergy and resulting cartilage formation remains unknown. To determine the optimal delivery method of a mixed population of stem cells and chondrocytes for cartilage regeneration, here we compared hydrogel and pellet co-culture on the interactions between adipose-derived stem cells (ADSCs) and neonatal chondrocytes (NChons). While both co-culture models supported synergy, hydrogel co-culture led to over a 5-fold increase in synergistic index in cell proliferation, and over 11 and 15-fold increases in synergistic indices for production of cartilage matrix (collagen and sGAG respectively). Interaction index analyses of gene expressions showed hydrogel co-culture significantly reduced hypertrophic phenotype of both ADSCs and NChons. In hydrogel co-culture, NChons contributed to the neocartilage deposition while ADSCs resided outside the newly formed cartilage nodule, serving as supporting cells through paracrine signaling. In contrast, during pellet co-culture, both NChons and ADSCs contributed to the newly formed cartilage. Using the same number of cells, hydrogel co-culture supported higher synergy and produced neocartilage tissues approximately 5 times the size of pellet co-culture. In contrast, pellet co-culture resulted in denser cartilage matrix suitable only for small defects. The outcomes of this study suggest hydrogel delivery as a more advantageous co-culture method for regenerating cartilage using mixed cell populations.
Lay Summary
Donor cell scarcity is a key barrier for clinical translation of cell-based therapies for cartilage regeneration. To overcome this challenge, here we harnessed abundantly available fat-derived stem cells and mixed them with juvenile chondrocytes, a cell type from native cartilage that can produce robust cartilage. We compared two different co-culture methods, hydrogel versus cell pellet, to grow the mixed cell populations for cartilage regeneration. We demonstrate that 3D hydrogels induced higher synergy using mixed cell populations, allowing cartilage repair with a reduced number of chondrocytes and defect filling of larger volumes than pellets.
Future Works
Future work will validate the potential of using hydrogels to deliver mixed population of fat-derived stem cells and juvenile chondrocytes for cartilage regeneration in vivo using relevant animal models.




Similar content being viewed by others
References
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–95.
Redman SN, Oldfield SF, Archer CW. Current strategies for articular cartilage repair. Eur Cell Mater. 2005;9:23–32 discussion 23-32.
Brittberg M, Tallheden T, Sjögren-Jansson E, Lindahl A, Peterson L. Autologous chondrocytes used for articular cartilage repair: an update. Clin Orthop Relat Res. 2001;391:S337–S48.
Grande DA, Pitman MI, Peterson L, Menche D, Klein M. The repair of experimentally produced defects in rabbit articular cartilage by autologous chondrocyte transplantation. J Orthop Res. 1989;7(2):208–18.
Saha S, Kirkham J, Wood D, Curran S, Yang X. Comparative study of the chondrogenic potential of human bone marrow stromal cells, neonatal chondrocytes and adult chondrocytes. Biochem Biophys Res Commun. 2010;401(3):333–8.
Adkisson HD, Martin JA, Amendola RL, Milliman C, Mauch KA, Katwal AB, et al. The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. Am J Sports Med. 2010;38(7):1324–33.
Adkisson HD, Milliman C, Zhang X, Mauch K, Maziarz RT, Streeter PR. Immune evasion by neocartilage-derived chondrocytes: implications for biologic repair of joint articular cartilage. Stem Cell Res. 2010;4(1):57–68.
Acharya C, Adesida A, Zajac P, Mumme M, Riesle J, Martin I, et al. Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. J Cell Physiol. 2012;227(1):88–97.
Wu L, Leijten JC, Georgi N, Post JN, van Blitterswijk CA, Karperien M. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng A. 2011;17(9–10):1425–36.
Wu L, Prins H-J, Helder MN, Blitterswijk CA, Karperien M. Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. Tissue Eng A. 2012;18(15–16):1542–51.
Hubka KM, Dahlin RL, Meretoja VV, Kasper FK, Mikos AG. Enhancing chondrogenic phenotype for cartilage tissue engineering: monoculture and coculture of articular chondrocytes and mesenchymal stem cells. Tissue Eng B Rev. 2014;20(6):641–54.
Meretoja VV, Dahlin RL, Kasper FK, Mikos AG. Enhanced chondrogenesis in co-cultures with articular chondrocytes and mesenchymal stem cells. Biomaterials. 2012;33(27):6362–9.
Bian L, Zhai DY, Mauck RL, Burdick JA. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng A. 2011;17(7–8):1137–45.
Lettry V, Hosoya K, Takagi S, Okumura M. Coculture of equine mesenchymal stem cells and mature equine articular chondrocytes results in improved chondrogenic differentiation of the stem cells. Jpn J Vet Res. 2010;58(1):5–15.
Lai JH, Rogan H, Kajiyama G, Goodman SB, Smith RL, Maloney W, et al. Interaction between osteoarthritic chondrocytes and adipose-derived stem cells is dependent on cell distribution in three-dimension and transforming growth factor-beta3 induction. Tissue Eng A. 2015;21(5–6):992–1002.
Lai JH, Kajiyama G, Smith RL, Maloney W, Yang F. Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Sci Rep. 2013;3.
Wang T, Lai JH, Han L-H, Tong X, Yang F. Modulating stem cell–chondrocyte interactions for cartilage repair using combinatorial extracellular matrix-containing hydrogels. J Mater Chem B. 2016;4(47):7641–50.
Tsuchiya K, Chen G, Ushida T, Matsuno T, Tateishi T. The effect of coculture of chondrocytes with mesenchymal stem cells on their cartilaginous phenotype in vitro. Mater Sci Eng C. 2004;24(3):391–6.
Ahmed N, Dreier R, Gopferich A, Grifka J, Grassel S. Soluble signalling factors derived from differentiated cartilage tissue affect chondrogenic differentiation of rat adult marrow stromal cells. Cell Physiol Biochem. 2007;20(5):665–78.
Estes BT, Diekman BO, Gimble JM, Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010;5(7):1294–311.
Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;290(2):763–9.
Guilak F, Awad HA, Fermor B, Leddy HA, Gimble JM. Adipose-derived adult stem cells for cartilage tissue engineering. Biorheology. 2004;41(3–4):389–99.
Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238(1):265–72.
Zhang L, Su P, Xu C, Yang J, Yu W, Huang D. Chondrogenic differentiation of human mesenchymal stem cells: a comparison between micromass and pellet culture systems. Biotechnol Lett. 2010;32(9):1339–46.
Wang T, Lai JH, Han LH, Tong X, Yang F. Chondrogenic differentiation of adipose-derived stromal cells in combinatorial hydrogels containing cartilage matrix proteins with decoupled mechanical stiffness. Tissue Eng A. 2014;20(15–16):2131–9.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28.
Fairbanks BD, Schwartz MP, Halevi AE, Nuttelman CR, Bowman CN, Anseth KS. A versatile synthetic extracellular matrix mimic via thiol-norbornene photopolymerization. Adv Mater. 2009;21(48):5005–10.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25(4):402–8.
Lee J, Smeriglio P, Chu CR, Bhutani N. Human iPSC-derived chondrocytes mimic juvenile chondrocyte function for the dual advantage of increased proliferation and resistance to IL-1beta. Stem Cell Res Ther. 2017;8(1):244.
Wagner DR, Lindsey DP, Li KW, Tummala P, Chandran SE, Smith RL, et al. Hydrostatic pressure enhances chondrogenic differentiation of human bone marrow stromal cells in osteochondrogenic medium. Ann Biomed Eng. 2008;36(5):813–20.
Funding
The authors acknowledge NIH R01DE024772 (F.Y.), NSF CAREER award CBET-1351289 (F.Y.), California Institute for Regenerative Medicine Tools and Technologies Award RT3-07804 (F.Y.), the Stanford Bio-X Interdisciplinary Initiative Seed grant (F.Y.), the Stanford Child Health Research Institute Faculty Scholar Award (F.Y.), NSF Graduate Research Fellowship Program (H.R.), and the Stanford Interdisciplinary Graduate Fellowship from the Stanford Bio-X program (H. R.) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 29 kb)
Rights and permissions
About this article
Cite this article
Rogan, H., Yang, F. Optimizing 3D Co-culture Models to Enhance Synergy Between Adipose-Derived Stem Cells and Chondrocytes for Cartilage Tissue Regeneration. Regen. Eng. Transl. Med. 5, 270–279 (2019). https://doi.org/10.1007/s40883-019-00105-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-019-00105-6