Abstract
Background
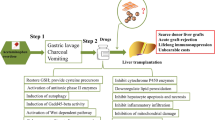
Liver injury poses to be a prevalent and persistent problem at a global scale and liver transplantation using umbilical cord blood–borne human mesenchymal stem cells (hMSCs) being the frequent respite to overcome it. Although there has been a plethora of advancements in liver transplantation studies, failures of a successful transplant remain difficult to evade, majorly due to lack of hMSC survivability at the injured site. Hence, in this study, the effect of delta opioid receptor (DOR)–activated hMSCs, reported to have shown a pronounced increase in hMSC survivability in vitro under different stress conditions, have been illustrated on an acute liver injury model of mice.
Methods
Acetaminophen, a commonly used paracetamol for induction of liver injury was administered intraperitoneally at a dosage of 500 mg/kg to the treatment groups of mice. The control groups included without treatment phosphate buffer saline (PBS) injected. For the transplantation of hMSCs, tail vein injection route of administration was followed at a dose of 5 × 105 cells/ animal. After 48 h, the liver tissues and blood samples were collected for determination of the ALT-AST activity and the alterations in the levels of inflammatory cytokines. Alongside, liver tissues were fixed using 10% formalin and observed for portal and lobular inflammation.
Results
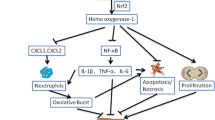
The transplantation of hMSCs prevented the increase in the levels of serum alanine transaminase and aspartate transaminase, respectively, in comparison with the acetaminophen-treated groups. There was an additional repression observed in their levels upon transplantation with DOR-activated hMSCs. Analysis of the inflammatory cytokines post-induction and transplantation of hMSCs and DOR-activated hMSCs revealed a prominent mitigation of the pro-inflammatory cytokines IL-1, IL-6, and TNF-α by over ~ 4-folds and a significant upregulation of anti-inflammatory cytokine IL-10 by about ~ 4-folds when compared to the acetaminophen-treated. Histological evidences of the liver tissue samples also followed a similar trend wherein maximum necrotic tissues were observed in the groups treated with acetaminophen. The groups transplanted with hMSCs showed potential recuperation from necrosis and inflammation, which were further curbed down in groups transplanted with DOR-activated hMSCs.
Conclusion
This study corroborates the potential benefits of transplanting DOR-activated hMSCs in a liver injury mice model and implies that recuperation of the mice groups with DOR-administered hMSCs was majorly due to the amelioration in the inflammatory cytokines, along with subdued levels of ALT-AST enzymes. Therefore, DOR activation on hMSCs could prove to be a successful prospective therapeutic for a repertoire of such liver failure models.


Similar content being viewed by others
References
Kim HJ, Park J-S. Usage of human mesenchymal stem cells in cell-based therapy: advantages and disadvantages. Dev Reprod. 2017;21:1–10.
Singh A, Singh A, Sen D. Mesenchymal stem cells in cardiac regeneration: a detailed progress report of the last 6 years (2010-2015). Stem Cell Res Ther. 2016;7:82.
Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117.
Faiella W, Atoui R. Immunotolerant properties of mesenchymal stem cells: updated review. Stem Cells Int. 2016;2016:1–7.
Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–8.
Kaminski N, Phinney DG, Baddoo M, Gambelli F, McBride C, Ortiz LA, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100(14):8407–11.
Yannarelli G, Tsoporis JN, Desjardins JF, Wang XH, Pourdjabbar A, Viswanathan S, et al. Donor mesenchymal stromal cells (MSCs) undergo variable cardiac reprogramming in vivo and predominantly co-express cardiac and stromal determinants after experimental acute myocardial infarction. Stem Cell Rev Rep. 2014;10:304–15.
Chapel A, Bertho JM, Bensidhoum M, Fouillard L, Young RG, Frick J, et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med. 2003;5:1028–38.
Yadav N, Kanjirakkuzhiyil S, Kumar S, Jain M, Halder A, Saxena R, et al. The therapeutic effect of bone marrow-derived liver cells in the phenotypic correction of murine hemophilia a. Blood. 2009;114:4552–61.
Mullick M, Venkatesh K, Sen D. D-alanine 2, leucine 5 enkephaline (DADLE)-mediated DOR activation augments human hUCB-BFs viability subjected to oxidative stress via attenuation of the UPR. Stem Cell Res. 2017;22:20–8.
Reddy LVK, Sen D. DADLE enhances viability and anti-inflammatory effect of human MSCs subjected to ‘serum free’ apoptotic condition in part via the DOR/PI3K/AKT pathway. Life Sci. 2017;191:195–204.
Mullick M, Sen D. The delta opioid peptide DADLE represses hypoxia-reperfusion mimicked stress mediated apoptotic cell death in human mesenchymal stem cells in part by downregulating the unfolded protein response and ROS along with enhanced anti-inflammatory effect. Stem Cell Rev Rep. 2018;14:558–73.
Zheng S, Yang J, Yang J, Tang Y, Shao Q, Guo L, et al. Transplantation of umbilical cord mesenchymal stem cells via different routes in rats with acute liver failure. Int J Clin Exp Pathol. 2015;8(12):15854–62.
Williams R, Schalm SW, O’Grady JG. Acute liver failure: redefining the syndromes. Lancet. 1993;342:273–5.
Hay JE. Acute liver failure. Curr Treat Options Gastroenterol. 2004;7:459–68.
Jaeschke H. Mechanisms of sterile inflammation in acetaminophen hepatotoxicity. Cell Mol Immunol. 2018;15:74–5.
Lawson J, Farhood A, Hopper R. The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol Sci. 2000;54:509–16.
Shen W, Kamendulis LM, Ray SD, Corcoran GB. Acetaminophen-induced cytotoxicity in cultured mouse hepatocytes: correlation of nuclear Ca2+ accumulation and early DNA fragmentation with cell death. Toxicol Appl Pharmacol. 1991;111:242–54.
Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41.
Gurule MW, Yorkin RD, Kamendulis LM, Ray SD, Corcoran GB. Ca2+ antagonists inhibit DNA fragmentation and toxic cell death induced by acetaminophen. FASEB J. 2018.
Jan YH, Heck DE, Dragomir AC, Gardner CR, Laskin DL, Laskin JD. Acetaminophen reactive intermediates target hepatic thioredoxin reductase. Chem Res Toxicol. 2014;27:882–94.
Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin N Am. 2008;92:761–94.
Gabriel N, Samuel R, Jayandharan GR. Targeted delivery of AAV-transduced mesenchymal stromal cells to hepatic tissue for ex vivo gene therapy. J Tissue Eng Regen Med. 2017;11:1354–64.
Begum MET, Sen D. DOR agonist (SNC-80) exhibits anti-parkinsonian effect via downregulating UPR/oxidative stress signals and inflammatory response in vivo. Neurosci Lett. 2018;678:29–36.
Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–16.
Park M, Kim YH, Woo SY, Lee HJ, Yu Y, Kim HS, et al. Tonsil-derived mesenchymal stem cells ameliorate CCl4-induced liver fibrosis in mice via autophagy activation. Sci Rep. 2015;5:8616.
Lawson JA. The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol Sci. 2002;54(2):509–16.
Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973;187(1):185–94.
Acknowledgements
The authors are grateful to Ms. Moghal Erfath Thanjeem Begum and Ms. Pearlin Hameed for their liberal support in handling and treatment procedures of the mice.
Funding
This study was funded by “SEED” grant from VIT, Vellore awarded to DS.
Author information
Authors and Affiliations
Contributions
MM has carried out experiments, analyzed data, and wrote the paper. SB has carried out experiments. DS conceptualized, wrote the paper, and analyzed the data.
Corresponding author
Ethics declarations
All animal experiments were reviewed and approved by the Institutional animal ethics committee of VIT, Vellore, India.
Consent for Publication
All the authors have read the manuscript and agreed to the publication.
Competing Interests
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mullick, M., Banerjee, S. & Sen, D. Amelioration of Acetaminophen-Induced Liver Injury Via Delta Opioid Receptor–Activated Human Mesenchymal Stem Cells—an In Vivo Approach. Regen. Eng. Transl. Med. 5, 263–269 (2019). https://doi.org/10.1007/s40883-019-00101-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-019-00101-w