Abstract
Background
Lack of angiogenesis, inflammation, and low differentiation of MSCs upon transplantation to ischemic site are major drawbacks in cell therapies for myocardial tissue regeneration. To overcome these problems, this study aimed to increase the angiogenesis, anti-inflammation, and differentiation of human umbilical cord MSCs (hMSCs) into cardiomyocytes by activating the delta opioid pathway by its agonist SNC80 with the combination of zebularine, activin A and oxytocin.
Materials and Methods
Human umbilical cord MSCs were seeded on gelatin-coated plates by using alpha MEM. MSCs were treated with SNC80 and the combination of zebularine, activin A and oxytocin. Media and inducers were changed every 3 days once until 21 days. Spent media collected from day 7 and day 21 was used to estimate angiogenic (VEGF) and anti- or pro-inflammatory cytokines (IL-10, IL1b, IL-6) levels by ELISA. The effect of spent media on in vitro endothelial tube formation and anti-inflammatory effect on inflammation induced with LPS on macrophages was also studied along with cytokine levels. Proteins that were isolated from day 7 and day 21 were used to study for cardiomyocyte markers (GATA4, MEF2C, connexin 43, and KCNJ2) and NOTCH1 involved in cardiomyocyte differentiation using Western blot.
Results
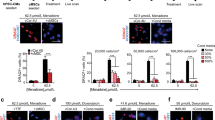
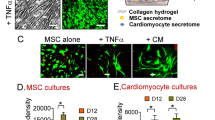
Activation of DOR increases the VEGF secretion levels estimated by ELISA and increases the in vitro tube formation in terms of number of loops and total length. Delta opioid receptor (DOR)-activated conditioned media secreted anti-inflammatory cytokine IL-10, which could prevent the secretion of pro-inflammatory cytokines IL-1b and IL-6 from LPS induced macrophages. DOR activated MSCs upregulated the early and late cardiomyocytes markers (GATA4, MEF2C and connexin 43, KCNJ43) via NOTCH1 signaling.
Conclusion
Activation of DOR on hMSCs plays a vital role in angiogenesis, anti-inflammation, and cardiomyocyte differentiation by regulating the NOTCH1 signaling pathway.
Lay Summary
Our results indicate that, upon activation of delta opioid receptors on human umbilical cord derived mesenchymal stem cells, they can differentiate into heart cells (by expressing specific markers GATA4, MEF2C, Connexin43), show enhanced formation of blood vessels and anti-inflammatory activity. This approach may help to treat pateints post heart attack.








Similar content being viewed by others
References
Tzahor E, Poss KD. Cardiac regeneration strategies: staying young at heart. Science. 2017;356(6342):1035–9.
Mullick M, Venkatesh K, Sen D. D-alanine 2, leucine 5 enkephaline (DADLE)-mediated DOR activation augments human hUCB-BFs viability subjected to oxidative stress via attenuation of the UPR. Stem Cell Res. 2017;22:20–8.
Mullick M, Sen D. The delta opioid peptide DADLE represses hypoxia-reperfusion mimicked stress mediated apoptotic cell death in human mesenchymal stem cells in part by downregulating the unfolded protein response and ROS along with enhanced anti-inflammatory effect. Stem Cell Rev. 2018;14(4):558–73.
Reddy LVK, Sen D. DADLE enhances viability and anti-inflammatory effect of human MSCs subjected to 'serum free' apoptotic condition in part via the DOR/PI3K/AKT pathway. Life Sci. 2017;191:195–204.
Bigliardi-Qi M, Gaveriaux-Ruff C, Zhou H, Hell C, Bady P, Rufli T, et al. Deletion of δ-opioid receptor in mice alters skin differentiation and delays wound healing. Differentiation. 2006;74(4):174–85.
Sinova R, Kudova J, Nesporova K, Karel S, Sulakova R, Velebny V, et al. Opioid receptors and opioid peptides in the cardiomyogenesis of mouse embryonic stem cells. J Cell Physiol 2018.
Stein C, Kuchler S. Targeting inflammation and wound healing by opioids. Trends Pharmacol Sci. 2013;34(6):303–12.
Shrivastava P, Cabrera MA, Chastain LG, Boyadjieva NI, Jabbar S, Franklin T, et al. Mu-opioid receptor and delta-opioid receptor differentially regulate microglial inflammatory response to control proopiomelanocortin neuronal apoptosis in the hypothalamus: effects of neonatal alcohol. J Neuroinflammation. 2017;14(1):83.
Yang G, Xiao Z, Ren X, Long H, Ma K, Qian H, et al. Obtaining spontaneously beating cardiomyocyte-like cells from adipose-derived stromal vascular fractions cultured on enzyme-crosslinked gelatin hydrogels. Sci Rep. 2017;7:41781.
Luxan G, D'Amato G, MacGrogan D, de la Pompa JL. Endocardial NOTCH signaling in cardiac development and disease. Circ Res. 2016;118(1):e1–e18.
Garcia-Concejo A, Jimenez-Gonzalez A, Rodriguez RE. Opioid and NOTCH signaling pathways are reciprocally regulated through miR- 29a and miR-212 expression. Biochim Biophys Acta Gen Subj. 2018;1862(12):2605–12.
Yu Z, Zou Y, Fan J, Li C, Ma L. Notch1 is associated with the differentiation of human bone marrowderived mesenchymal stem cells to cardiomyocytes. Mol Med Rep. 2016;14(6):5065–71.
Muller-Ehmsen J, Krausgrill B, Burst V, Schenk K, Neisen UC, Fries JW, et al. Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J Mol Cell Cardiol. 2006;41(5):876–84.
Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93–8.
Pagani FD, DerSimonian H, Zawadzka A, Wetzel K, Edge AS, Jacoby DB, et al. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans. Histological analysis of cell survival and differentiation. J Am Coll Cardiol. 2003;41(5):879–88.
Khanabdali R, Saadat A, Fazilah M, Bazli KF, Qazi RE, Khalid RS, et al. Promoting effect of small molecules in cardiomyogenic and neurogenic differentiation of rat bone marrow-derived mesenchymal stem cells. Drug Des Devel Ther. 2015;10:81–91.
Kim YS, Ahn Y, Kwon JS, Cho YK, Jeong MH, Cho JG, et al. Priming of mesenchymal stem cells with oxytocin enhances the cardiac repair in ischemia/reperfusion injury. Cells Tissues Organs. 2012;195(5):428–42.
Qiao Y, Tang C, Wang Q, Wang D, Yan G, Zhu B. Kir2.1 regulates rat smooth muscle cell proliferation, migration, and post-injury carotid neointimal formation. Biochem Biophys Res Commun. 2016;477(4):774–80.
Lai PF, Panama BK, Masse S, Li G, Zhang Y, Kusha M, et al. Mesenchymal stem cell transplantation mitigates electrophysiological remodeling in a rat model of myocardial infarction. J Cardiovasc Electrophysiol. 2013;24(7):813–21.
Smith PD, Brett SE, Luykenaar KD, Sandow SL, Marrelli SP, Vigmond EJ, et al. KIR channels function as electrical amplifiers in rat vascular smooth muscle. J Physiol. 2008;586(4):1147–60.
Xia Y, Gong K-Z, Xu M, Zhang Y-Y, Guo J-h, Song Y, et al. Regulation of gap-junction protein connexin 43 by Î2-adrenergic receptor stimulation in rat cardiomyocytes. Acta Pharmacol Sin. 2009;30:928–34.
Yang Y, Yan X, Xue J, Zheng Y, Chen M, Sun Z, Liu T., Wang C., You H., Luo D. Connexin43 dephosphorylation at serine 282 is associated with connexin43-mediated cardiomyocyte apoptosis. Cell Death Differ 2019.
Mureli S, Gans CP, Bare DJ, Geenen DL, Kumar NM, Banach K. Mesenchymal stem cells improve cardiac conduction by upregulation of connexin 43 through paracrine signaling. Am J Physiol Heart Circ Physiol. 2012;304(4):H600–9.
Chen CC, Kuo CY, Chen RF. Role of CAPE on cardiomyocyte protection via connexin 43 regulation under hypoxia. Int J Med Sci. 2016;13(10):754–8.
Zhou XL, Liu JC. Role of Notch signaling in the mammalian heart. Braz J Med Biol Res. 2014;47(1):1–10.
Yamamizu K, Furuta S, Katayama S, Narita M, Kuzumaki N, Imai S, et al. The kappa opioid system regulates endothelial cell differentiation and pathfinding in vascular development. Blood. 2011;118(3):775–85.
Li Y, Hiroi Y, Liao JK. Notch signaling as an important mediator of cardiac repair and regeneration after myocardial infarction. Trends Cardiovasc Med. 2010;20(7):228–31.
Iwaszkiewicz K, Schneider J, Hua S. Targeting peripheral opioid receptors to promote analgesic and anti-inflammatory actions. Front Pharmacol [Mini Review]. 2013;4:132.
Funding
This study was funded by VIT SEED Fund awarded to DS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Reddy, L.V.K., Sen, D. Regulation of Cardiomyocyte Differentiation, Angiogenesis, and Inflammation by the Delta Opioid Signaling in Human Mesenchymal Stem Cells. Regen. Eng. Transl. Med. 5, 252–262 (2019). https://doi.org/10.1007/s40883-019-00100-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-019-00100-x