Abstract
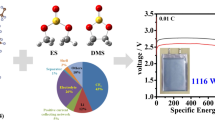
Lithium-fluorinated carbon (Li-CFx) batteries have become one of the most widely applied power sources for high energy density applications because of the advantages provided by the CFx cathode. Moreover, the large gap between the practical and theoretical potentials alongside the stoichiometric limit of commercial graphite fluorides indicates the potential for further energy improvement. Herein, monolayer fluorinated graphene nanoribbons (F-GNRs) were fabricated by unzipping single-walled carbon nanotubes (SWCNTs) using pure F2 gas at high temperature, which delivered an unprecedented energy density of 2738.45 W h kg−1 due to the combined effect of a high fluorination degree and discharge plateau, realized by the abundant edges and destroyed periodic structure, respectively. Furthermore, at a high fluorination temperature, the theoretical calculation confirmed a zigzag pathway of fluorine atoms that were adsorbed outside of the SWCNTs and hence initiated the spontaneous process of unzipping SWCNTs to form the monolayer F-GNRs. The controllable fluorination of SWCNTs provided a feasible approach for preparing CFx compounds for different applications, especially for ultrahigh energy density cathodes.

摘要
由于CFx正极材料的优势, 锂-氟化碳(Li-CFx)电池已成为一 种应用广泛、能提供巨大能量密度的电源之一. 但其实际放电电 压与理论放电电压之间的较大差距, 以及商用氟化石墨的化学计 量极限, 使得进一步提高其能量密度面临挑战. 本文采用纯F2气体 直接在高温下对单壁碳纳米管(SWCNTs)进行切割, 制备出单层氟 化石墨烯纳米带(F-GNRs). 丰富的边缘结构和碳骨架周期性结构 的破坏使其具有高的氟化程度和放电平台, 从而使其能量密度高 达2738.45 W h kg−1. 理论计算表明在高氟化温度下, 氟原子在碳纳 米管外以zigzag路径吸附, 进一步证实了切割单壁碳纳米管形成单 层F-GNRs为自发过程. 单壁碳纳米管的可控氟化为制备具有不同 用途的CFx, 特别是具有超高能量密度正极材料提供了一条可行途 径.
Article PDF
Similar content being viewed by others
References
Krause FC, Jones JP, Jones SC, et al. High specific energy lithium primary batteries as power sources for deep space exploration. J Electrochem Soc, 2018, 165: A2312–A2320
Zhang T, Li Z, Hou W, et al. Nanomaterials for implantable batteries to power cardiac devices. Mater Today Nano, 2020, 9: 100070
Zhang Q, Takeuchi KJ, Takeuchi ES, et al. Progress towards high-power Li/CFx batteries: electrode architectures using carbon nanotubes with CFx. Phys Chem Chem Phys, 2015, 17: 22504–22518
Choi JW, Aurbach D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat Rev Mater, 2016, 1: 16013
Hartmann P, Bender CL, Vračar M, et al. A rechargeable room-temperature sodium superoxide (NaO2) battery. Nat Mater, 2013, 12: 228–232
Ren X, Wu Y. A low-overpotential potassium-oxygen battery based on potassium superoxide. J Am Chem Soc, 2013, 135: 2923–2926
Zhang Z, Zhang Q, Chen Y, et al. The first introduction of graphene to rechargeable Li-CO2 batteries. Angew Chem Int Ed, 2015, 54: 6550–6553
Qiao Y, Yi J, Wu S, et al. Li-CO2 electrochemistry: A new strategy for CO2 fixation and energy storage. Joule, 2017, 1: 359–370
He M, Li Y, Guo R, et al. Electrochemical conversion of nitrogen trifluoride as a gas-to-solid cathode in Li batteries. J Phys Chem Lett, 2018, 9: 4700–4706
Li Y, Khurram A, Gallant BM. A high-capacity lithium-gas battery based on sulfur fluoride conversion. J Phys Chem C, 2018, 122: 7128–7138
Gao H, Li Y, Guo R, et al. Controlling fluoride-forming reactions for improved rate capability in lithium-perfluorinated gas conversion batteries. Adv Energy Mater, 2019, 9: 1900393
He H, Ren F, Zhu J, et al. Highly-efficient conversion of SF6via an eight-electron transfer process in lithium batteries. Nano Energy, 2020, 72: 104679
Ma Y, Zhang H, Wu B, et al. Lithium sulfur primary battery with super high energy density: based on the cauliflower-like structured C/S cathode. Sci Rep, 2015, 5: 14949
Cheng JJ, Liu LF, Ou SW, et al. Sulfur/CuxS hybrid material for Li/S primary battery with improved discharge capacity. Mater Chem Phys, 2019, 224: 384–388
Zhan L, Song Z, Shan N, et al. Poly(tetrahydrobenzodithiophene): High discharge specific capacity as cathode material for lithium batteries. J Power Sources, 2009, 193: 859–863
Lu Y, Hou X, Miao L, et al. Cyclohexanehexone with ultrahigh capacity as cathode materials for lithium-ion batteries. Angew Chem Int Ed, 2019, 58: 7020–7024
Sun P, Bai P, Chen Z, et al. A lithium-organic primary battery. Small, 2020, 16: 1906462
Jones JP, Jones SC, Billings KJ, et al. Radiation effects on lithium CFx batteries for future spacecraft and landers. J Power Sources, 2020, 471: 228464
Peng C, Li Y, Yao F, et al. Ultrahigh-energy-density fluorinated calcinated macadamia nut shell cathodes for lithium/fluorinated carbon batteries. Carbon, 2019, 153: 783–791
Zhou R, Li Y, Feng Y, et al. The electrochemical performances of fluorinated hard carbon as the cathode of lithium primary batteries. Compos Commun, 2020, 21: 100396
Ahmad Y, Dubois M, Guérin K, et al. Pushing the theoretical limit of Li-CFx batteries using fluorinated nanostructured carbon nanodiscs. Carbon, 2015, 94: 1061–1070
Ahmad Y, Dubois M, Guerin K, et al. High energy density of primary lithium batteries working with sub-fluorinated few walled carbon nanotubes cathode. J Alloys Compd, 2017, 726: 852–859
Read J, Collins E, Piekarski B, et al. LiF formation and cathode swelling in the Li/CFx battery. J Electrochem Soc, 2011, 158: A504
Feng W, Long P, Feng Y, et al. Two-dimensional fluorinated graphene: synthesis, structures, properties and applications. Adv Sci, 2016, 3: 1500413
Sun C, Feng Y, Li Y, et al. Solvothermally exfoliated fluorographene for high-performance lithium primary batteries. Nanoscale, 2014, 6: 2634–2641
Zhong G, Chen H, Huang X, et al. High-power-density, high-energy-density fluorinated graphene for primary lithium batteries. Front Chem, 2018, 6: 50
Jiao L, Zhang L, Wang X, et al. Narrow graphene nanoribbons from carbon nanotubes. Nature, 2009, 458: 877–880
Rzeszotarski B, Mreńca-Kolasińska A, Szafran B. Electron spin inversion in fluorinated graphene nanoribbons. Phys Rev B, 2017, 96: 245307
Nguyen DK, Lin YT, Lin SY, et al. Fluorination-enriched electronic and magnetic properties in graphene nanoribbons. Phys Chem Chem Phys, 2017, 19: 20667–20676
Romero Aburto R, Alemany LB, Weldeghiorghis TK, et al. Chemical makeup and hydrophilic behavior of graphene oxide nanoribbons after low-temperature fluorination. ACS Nano, 2015, 9: 7009–7018
Yang Z, Liu M, Zhang C, et al. Carbon nanotubes bridged with graphene nanoribbons and their use in high-efficiency dye-sensitized solar cells. Angew Chem Int Ed, 2013, 52: 3996–3999
Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett, 1996, 77: 3865–3868
Blöchl PE. Projector augmented-wave method. Phys Rev B, 1994, 50: 17953–17979
Monkhorst HJ, Pack JD. Special points for Brillouin-zone integrations. Phys Rev B, 1976, 13: 5188–5192
Mickelson ET, Huffman CB, Rinzler AG, et al. Fluorination of single-wall carbon nanotubes. Chem Phys Lett, 1998, 296: 188–194
Lee YS, Cho TH, Lee BK, et al. Surface properties of fluorinated single-walled carbon nanotubes. J Fluorine Chem, 2003, 120: 99–104
Li Y, Feng Y, Feng W. Deeply fluorinated multi-wall carbon nanotubes for high energy and power densities lithium/carbon fluorides battery. Electrochim Acta, 2013, 107: 343–349
Park KA, Choi YS, Lee YH, et al. Atomic and electronic structures of fluorinated single-walled carbon nanotubes. Phys Rev B, 2003, 68: 045429
Marcoux PR, Schreiber J, Batail P, et al. A spectroscopic study of the fluorination and defluorination reactions on single-walled carbon nanotubes. Phys Chem Chem Phys, 2002, 4: 2278–2285
Jiao L, Wang X, Diankov G, et al. Facile synthesis of high-quality graphene nanoribbons. Nat Nanotech, 2010, 5: 321–325
Gu Z, Peng H, Hauge RH, et al. Cutting single-wall carbon nanotubes through fluorination. Nano Lett, 2002, 2: 1009–1013
Zhang P, Zhao F, Long P, et al. Sonication-assisted liquid-phase exfoliated α-GeTe: a two-dimensional material with high Fe3+ sensitivity. Nanoscale, 2018, 10: 15989–15997
Zbořil R, Karlický F, Bourlinos AB, et al. Graphene fluoride: A stable stoichiometric graphene derivative and its chemical conversion to graphene. Small, 2010, 6: 2885–2891
Alemany LB, Zhang L, Zeng L, et al. Solid-state NMR analysis of fluorinated single-walled carbon nanotubes: assessing the extent of fluorination. Chem Mater, 2007, 19: 735–744
Zhang SS, Foster D, Wolfenstine J, et al. Electrochemical characteristic and discharge mechanism of a primary Li/CFx cell. J Power Sources, 2009, 187: 233–237
Giraudet J, Delabarre C, Guérin K, et al. Comparative performances for primary lithium batteries of some covalent and semicovalent graphite fluorides. J Power Sources, 2005, 158: 1365–1372
Yazami R, Hamwi A, Guérin K, et al. Fluorinated carbon nanofibres for high energy and high power densities primary lithium batteries. Electrochem Commun, 2007, 9: 1850–1855
Dai Y, Fang Y, Cai S, et al. Surface modified pinecone shaped hierarchical structure fluorinated mesocarbon microbeads for ultrafast discharge and improved electrochemical performances. J Electrochem Soc, 2017, 164: A1–A7
Wang L, Li Y, Wang S, et al. Fluorinated nanographite as a cathode material for lithium primary batteries. ChemElectroChem, 2019, 6: 2201–2207
Xia C, Kwok CY, Nazar LF. A high-energy-density lithium-oxygen battery based on a reversible four-electron conversion to lithium oxide. Science, 2018, 361: 777–781
Acknowledgements
This work was financially supported by the National Key R&D Program of China (2016YFA0202302), the State Key Program of National Natural Science Foundation of China (51633007), and the National Natural Science Foundation of China (51773147, 51803149 and 51973151).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding authors
Additional information
Conflict of interest
The authors declare no conflict of interest.
Cong Peng obtained his MSc degree from Hubei University in 2016. He is currently working on his PhD in Professor Feng’s group at the School of Materials Science and Engineering in Tianjin University. His research interests include the synthesis, assemblies, and application of fluorinated carbon materials.
Yu Li is a lecturer at the School of Materials Science and Engineering, Tianjin University. He obtained his PhD degree from Tianjin University in 2011 and had worked as a postdoctoral research fellow at Tianjin University from 2011 to 2014. His current research is focused on conductive polymers and nano energy storage materials.
Wei Feng received his PhD in 2000 from Xi’an Jiaotong University focusing on photoelectric properties and device applications of novel conducting polymers. Then he worked at Osaka University and Tsinghua University as a JSPS fellow and postdoctoral researcher, respectively. In 2004, he became a full professor at Tianjin University. His research interest is the functional nanocarbon materials.
Supplementary Material
40843_2020_1551_MOESM2_ESM.pdf
Fluorinated graphene nanoribbons from unzipped single-walled carbon nanotubes for ultrahigh energy density lithium-fluorinated carbon batteries
Rights and permissions
About this article
Cite this article
Peng, C., Kong, L., Li, Y. et al. Fluorinated graphene nanoribbons from unzipped single-walled carbon nanotubes for ultrahigh energy density lithium-fluorinated carbon batteries. Sci. China Mater. 64, 1367–1377 (2021). https://doi.org/10.1007/s40843-020-1551-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-020-1551-x