Abstract
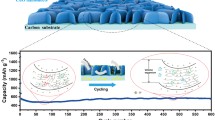
The robust porous architectures of active materials are highly desired for oxygen electrodes in lithium–oxygen batteries to enable high capacities and excellent reversibility. Herein, we report a novel three-dimensional replication strategy to fabricate three-dimensional architecture of porous carbon for oxygen electrodes in lithium–oxygen batteries. As a demonstration, ball-flower-like carbon microspheres assembled with tortuous hollow carbon nanosheets are successfully prepared by completely replicating the morphology of the nanostructured zinc oxide template and utilizing the polydopamine coating layer as the carbon source. When used as the active material for oxygen electrodes, the three-dimensional porous architecture of the prepared ballflower- like carbon microspheres can accommodate the discharge product lithium peroxide and simultaneously maintain the ions and gas diffusion paths. Moreover, their high degrees of defectiveness by nitrogen doping provide sufficient active sites for oxygen reduction/evolution reaction. Thus the prepared ball-flower-like carbon microspheres demonstrate a high capacity of 9,163.7 mA h g−1 and excellent reversibility. This work presents an effective way to prepare three-dimensional architectures of porous carbon by replicating the controllable nanostructures of transition metal oxide templates for energy storage and conversion applications.
Abstract
活性物质和电极的多孔结构设计是实现锂氧电池中氧气电极高容量和良好可逆性的关键措施. 本文报道了一种创新的三维复刻策 略, 并用于设计锂氧电池氧气电极用多孔碳材料的三维结构. 作为一个实例, 采用聚多巴胺包覆层为碳源, 通过完整复制纳米结构氧化锌 模板的形貌, 成功地制备了由扭曲的中空碳纳米片组装而成的花球状碳微球. 作为氧气电极的活性物质, 花球状碳微球的三维多孔结构不 仅能容纳放电产物过氧化锂, 同时也能保持离子和气体的扩散通道. 此外, 氮掺杂引入的高缺陷为氧还原/析出反应提供了充足的活性位 点. 从而, 花球状碳微球表现出高达9163.7 mA h g−1的比容量和优异的可逆性. 本工作呈现了一种用于能源存储和转化的多孔碳材料的三 维结构的有效可控制备方法, 即复制过渡金属氧化物模板的纳米结构.
Article PDF
Similar content being viewed by others
References
Lu J, Li L, Park JB, et al. Aprotic and aqueous Li–O2 batteries. Chem Rev, 2014, 114: 5611–5640
Wang L, Zhang Y, Liu Z, et al. Understanding oxygen electrochemistry in aprotic Li–O2 batteries. Green Energy Environ, 2017, 2: 186–203
Feng N, He P, Zhou H. Critical challenges in rechargeable aprotic Li-O2 batteries. Adv Energy Mater, 2016, 6: 1502303
Aurbach D, McCloskey BD, Nazar LF, et al. Advances in understanding mechanisms underpinning lithium–air batteries. Nat Energy, 2016, 1: 16128
Adams BD, Radtke C, Black R, et al. Current density dependence of peroxide formation in the Li–O2 battery and its effect on charge. Energy Environ Sci, 2013, 6: 1772–1778
Peng Z, Freunberger SA, Chen Y, et al. A reversible and higherrate Li-O2 battery. Science, 2012, 337: 563–566
Viswanathan V, Thygesen KS, Hummelshøj JS, et al. Electrical conductivity in Li2O2 and its role in determining capacity limitations in non-aqueous Li-O2 batteries. J Chem Phys, 2011, 135: 214704
Liu Y, He P, Zhou H. Rechargeable solid-state Li-air and Li-S batteries: Materials, construction, and challenges. Adv Energy Mater, 2018, 8: 1701602
Song K, Agyeman DA, Jung J, et al. A review of the design strategies for tailored cathode catalyst materials in rechargeable Li- O2 batteries. Isr J Chem, 2015, 55: 458–471
Ma Z, Yuan X, Li L, et al. A review of cathode materials and structures for rechargeable lithium–air batteries. Energy Environ Sci, 2015, 8: 2144–2198
Yin YB, Xu JJ, Liu QC, et al. Macroporous interconnected hollow carbon nanofibers inspired by golden-toad eggs toward a binderfree, high-rate, and flexible electrode. Adv Mater, 2016, 28: 7494–7500
Guo Z, Zhou D, Dong XL, et al. Ordered hierarchical mesoporous/macroporous carbon: a high-performance catalyst for rechargeable Li-O2 batteries. Adv Mater, 2013, 25: 5668–5672
Shu C, Li B, Zhang B, et al. Hierarchical nitrogen-doped graphene/carbon nanotube composite cathode for lithium-oxygen batteries. ChemSusChem, 2015, 8: 3973–3976
Sun B, Huang X, Chen S, et al. Porous graphene nanoarchitectures: an efficient catalyst for low charge-overpotential, long life, and high capacity lithium–oxygen batteries. Nano Lett, 2014, 14: 3145–3152
Lu J, Lei Y, Lau KC, et al. A nanostructured cathode architecture for low charge overpotential in lithium-oxygen batteries. Nat Commun, 2013, 4: 2383
Franco AA, Xue KH. Carbon-based electrodes for lithium air batteries: Scientific and technological challenges from a modeling perspective. ECS J Solid State Sci Technol, 2013, 2: M3084–M3100
Tran C, Yang XQ, Qu D. Investigation of the gas-diffusionelectrode used as lithium/air cathode in non-aqueous electrolyte and the importance of carbon material porosity. J Power Sources, 2010, 195: 2057–2063
Yang X, He P, Xia Y. Preparation of mesocellular carbon foam and its application for lithium/oxygen battery. Electrochem Commun, 2009, 11: 1127–1130
Nie H, Zhang H, Zhang Y, et al. Nitrogen enriched mesoporous carbon as a high capacity cathode in lithium–oxygen batteries. Nanoscale, 2013, 5: 8484–8487
Kang J, Li OL, Saito N. Hierarchical meso–macro structure porous carbon black as electrode materials in Li–air battery. J Power Sources, 2014, 261: 156–161
Xiao J, Mei D, Li X, et al. Hierarchically porous graphene as a lithium–air battery electrode. Nano Lett, 2011, 11: 5071–5078
Wang ZL, Xu D, Xu JJ, et al. Graphene oxide gel-derived, freestanding, hierarchically porous carbon for high-capacity and highrate rechargeable Li-O2 batteries. Adv Funct Mater, 2012, 22: 3699–3705
Lin Y, Moitoso B, Martinez-Martinez C, et al. Ultrahigh-capacity lithium–oxygen batteries enabled by dry-pressed holey graphene air cathodes. Nano Lett, 2017, 17: 3252–3260
McCloskey BD, Speidel A, Scheffler R, et al. Twin problems of interfacial carbonate formation in nonaqueous Li–O2 batteries. J Phys Chem Lett, 2012, 3: 997–1001
Ottakam Thotiyl MM, Freunberger SA, Peng Z, et al. The carbon electrode in nonaqueous Li–O2 cells. J Am Chem Soc, 2013, 135: 494–500
Yao X, Dong Q, Cheng Q, et al. Why do lithium-oxygen batteries fail: Parasitic chemical reactions and their synergistic effect. Angew Chem Int Ed, 2016, 55: 11344–11353
Sun B, Chen S, Liu H, et al. Mesoporous carbon nanocube architecture for high-performance lithium-oxygen batteries. Adv Funct Mater, 2015, 25: 4436–4444
Liu Y, Ai K, Lu L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem Rev, 2014, 114: 5057–5115
Xiao L, Mei D, Cao M, et al. Effects of structural patterns and degree of crystallinity on the performance of nanostructured ZnO as anode material for lithium-ion batteries. J Alloys Compd, 2015, 627: 455–462
Kuo CL, Kuo TJ, Huang MH. Hydrothermal synthesis of ZnO microspheres and hexagonal microrods with sheetlike and platelike nanostructures. J Phys Chem B, 2005, 109: 20115–20121
Kaneko K. Determination of pore size and pore size distribution. J Membrane Sci, 1994, 96: 59–89
Zhang Y, Zhang H, Li J, et al. The use of mixed carbon materials with improved oxygen transport in a lithium-air battery. J Power Sources, 2013, 240: 390–396
Horstmann B, Gallant B, Mitchell R, et al. Rate-dependent morphology of Li2O2 growth in Li–O2 batteries. J Phys Chem Lett, 2013, 4: 4217–4222
Kichambare P, Kumar J, Rodrigues S, et al. Electrochemical performance of highly mesoporous nitrogen doped carbon cathode in lithium–oxygen batteries. J Power Sources, 2011, 196: 3310–3316
Xie J, Yao X, Cheng Q, et al. Three dimensionally ordered mesoporous carbon as a stable, high-performance Li-O2 battery cathode. Angew Chem Int Ed, 2015, 54: 4299–4303
Acknowledgement
This work was supported by grants from the National Natural Science Foundation of China (21673169 and 51672205), the National Key R&D Program of China (2016YFA0202602), the Research Start-Up Fund from Wuhan University of Technology, and the Fundamental Research Funds for the Central Universities (WUT: 2017IB005, 2016IVA083).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Liang Xiao received his PhD degree in Physical Chemistry from Wuhan University. He worked at Pacific Northwest National Laboratory as a visiting scholar for one year from 2013 to 2014. He is currently a Professor at the School of Chemistry, Chemical Engineering and Life Sciences, Wuhan University of Technology. His current research focuses on nanostructured materials for lithium ion batteries and metal air batteries.
Jinping Liu received his PhD degree from Central China Normal University in June 2009. During 2008–2011, he did visiting and post-doctoral research at Nanyang Technological University in Singapore. He is currently Chair Professor at Wuhan University of Technology. His research interest includes the synthesis and electrochemical applications (batteries, supercapacitors & electrocatalysis) of nanostructures.
Electronic supplementary material
40843_2018_9367_MOESM1_ESM.pdf
Ball-flower-like carbon microspheres via a three-dimensional replication strategy as a high-capacity cathode in lithium–oxygen batteries
Rights and permissions
About this article
Cite this article
Xiao, L., Yi, J., Meng, W. et al. Ball-flower-like carbon microspheres via a three-dimensional replication strategy as a high-capacity cathode in lithium–oxygen batteries. Sci. China Mater. 62, 633–644 (2019). https://doi.org/10.1007/s40843-018-9367-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-018-9367-3