Abstract
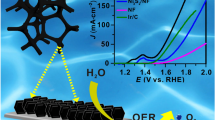
Clean energy technologies such as water splitting and fuel cells have been intensively pursued in the last decade for their free pollution. However, there is plenty of fossil energy consumed in the preparation of the catalysts, which results in a heavy pollution. Therefore, it is much desired but challenging to fabricate high-efficiency catalysts without extra energy input. Herein, we used a facile one-pot room-temperature method to synthesize a highly efficient electrocatalyst of nickel iron layered double hydroxide grown on Ni foam (NiFe LDH/NF) for oxygen evolution reaction (OER). The formation of the NiFe LDH follows a dissolution-precipitation process, in which the acid conditions by hydrolysis of Fe3+ combined with NO3− could etch the NF to form Ni2+. Then, the obtained Ni2+ was co-precipitated with the hydrolysed Fe3+ to in situ generate NiFe LDH on the NF. The NiFe LDH/NF exhibits excellent OER performance with a low potential of about 1.411 V vs. reversible hydrogen electrode (RHE) at a current density of 10 mA cm−2, a small Tafel slope of 42.3 mV dec−1 and a significantly low potential of ~1.452 V vs. RHE at 100 mA cm−2 in 1 mol L−1 KOH. Moreover, the material also keeps its original morphology and structure over 20 h. This energy-efficient strategy to synthesize NiFe LDH is highly promising for widespread application in OER catalyst industry.
摘要
绿色能源技术如电解水和燃料电池等由于其无污染的特点, 近年来一直受到人们的广泛关注. 然而, 在合成其催化剂的过程中多会 消耗化石能源, 从而造成环境污染, 形成恶性循环. 因此, 在无额外能量输入的条件下合成高效的电催化剂是非常必要的, 但同时又充满挑 战. 本文通过简单的一步合成法在室温下制备了一种具有高效析氧催化性能的镍铁层状双氢氧化物/泡沫镍(NiFe LDH/NF)催化剂. NiFe LDH的形成遵循溶解-沉淀机理: Fe3+水解产生的酸性环境联合NO3−, 刻蚀泡沫镍表面, 形成Ni2+, 随后, Ni2+与水解的Fe物种原位共沉淀于 泡沫镍表面, 生成NiFe LDH. 所得到的NiFe LDH/NF在碱性环境下, 表现出高效的电催化析氧反应性能. 在1 mol L−1的氢氧化钾溶液中, 当 电流密度为10 mA cm−2时, 其电位低至1.411 V vs. RHE, 相应的塔菲尔斜率仅为42.3 mV dec−1, 而在电流密度为100 mA cm−2时, 所需电位 也仅为1.452 V vs. RHE. 此外, 该材料还表现出卓越的结构稳定性. 这种绿色制备NiFe LDH/NF的合成方法有望在OER催化中得到广泛的 应用.
Similar content being viewed by others
References
Holladay JD, Hu J, King DL, et al. An overview of hydrogen production technologies. Catal Today, 2009, 139: 244–260
Winter M, Brodd RJ. What are batteries, fuel cells, and supercapacitors? Chem Rev, 2004, 104: 4245–4270
Gong M, Zhou W, Tsai MC, et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat Commun, 2014, 5: 4695
Suntivich J, May KJ, Gasteiger HA, et al. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science, 2011, 334: 1383–1385
Liang Y, Li Y, Wang H, et al. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat Mater, 2011, 10: 780–786
Jiao F, Frei H. Nanostructured cobalt and manganese oxide clusters as efficient water oxidation catalysts. Energy Environ Sci, 2010, 3: 1018–1027
Yagi M, Kaneko M. Molecular catalysts for water oxidation. Chem Rev, 2001, 101: 21–36
Gong M, Dai H. A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res, 2014, 8: 23–39
Kanan MW, Nocera DG. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science, 2008, 321: 1072–1075
Han X, Yu C, Zhou S, et al. Ultrasensitive iron-triggered nanosized Fe-CoOOH integrated with graphene for highly efficient oxygen evolution. Adv Energy Mater, 2017, 7: 1602148
Jiang Q, Xu L, Chen N, et al. Facile synthesis of black phosphorus: an efficient electrocatalyst for the oxygen evolving reaction. Angew Chem Int Ed, 2016, 55: 13849–13853
Yang Y, Zhang K, Lin H, et al. MoS2–Ni3S2 heteronanorods as efficient and stable bifunctional electrocatalysts for overall water splitting. ACS Catal, 2017, 7: 2357–2366
Xie J, Zhang J, Li S, et al. Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. J Am Chem Soc, 2013, 135: 17881–17888
Zou X, Zhang Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem Soc Rev, 2015, 44: 5148–5180
Lin L, Zhu Q, Xu AW. Noble-metal-free Fe–N/C catalyst for highly efficient oxygen reduction reaction under both alkaline and acidic conditions. J Am Chem Soc, 2014, 136: 11027–11033
Gewirth AA, Varnell JA, DiAscro AM. Nonprecious metal catalysts for oxygen reduction in heterogeneous aqueous systems. Chem Rev, 2018, 118: 2313–2339
Sivanantham A, Ganesan P, Shanmugam S. Hierarchical NiCo2S4 nanowire arrays supported on Ni foam: an efficient and durable bifunctional electrocatalyst for oxygen and hydrogen evolution reactions. Adv Funct Mater, 2016, 26: 4661–4672
Li Y, Hasin P, Wu Y. NixCo3−xO4 nanowire arrays for electrocatalytic oxygen evolution. Adv Mater, 2010, 22: 1926–1929
Gorlin Y, Jaramillo TF. A bifunctional nonprecious metal catalyst for oxygen reduction and water oxidation. J Am Chem Soc, 2010, 132: 13612–13614
Cui B, Lin H, Li JB, et al. Core-ring structured NiCo2O4 nanoplatelets: synthesis, characterization, and electrocatalytic applications. Adv Funct Mater, 2008, 18: 1440–1447
Zhang J, Liu J, Xi L, et al. Single-atom Au/NiFe layered double hydroxide electrocatalyst: probing the origin of activity for oxygen evolution reaction. J Am Chem Soc, 2018, 140: 3876–3879
Wu L, Li Q, Wu CH, et al. Stable cobalt nanoparticles and their monolayer array as an efficient electrocatalyst for oxygen evolution reaction. J Am Chem Soc, 2015, 137: 7071–7074
Zou X, Huang X, Goswami A, et al. Cobalt-embedded nitrogenrich carbon nanotubes efficiently catalyze hydrogen evolution reaction at all pH values. Angew Chem Int Ed, 2014, 53: 4372–4376
Song F, Hu X. Ultrathin cobalt–manganese layered double hydroxide is an efficient oxygen evolution catalyst. J Am Chem Soc, 2014, 136: 16481–16484
Trotochaud L, Young SL, Ranney JK, et al. Nickel–iron oxyhydroxide oxygen-evolution electrocatalysts: the role of intentional and incidental iron incorporation. J Am Chem Soc, 2014, 136: 6744–6753
Qi J, Zhang W, Xiang R, et al. Porous nickel-iron oxide as a highly efficient electrocatalyst for oxygen evolution reaction. Adv Sci, 2015, 2: 1500199
Jiang J, Zhang C, Ai L. Hierarchical iron nickel oxide architectures derived from metal-organic frameworks as efficient electrocatalysts for oxygen evolution reaction. Electrochim Acta, 2016, 208: 17–24
Jiang J, Lu S, Wang WK, et al. Ultrahigh electrocatalytic oxygen evolution by iron-nickel sulfide nanosheets/reduced graphene oxide nanohybrids with an optimized autoxidation process. Nano Energy, 2018, 43: 300–309
Song B, Li K, Yin Y, et al. Tuning mixed nickel iron phosphosulfide nanosheet electrocatalysts for enhanced hydrogen and oxygen evolution. ACS Catal, 2017, 7: 8549–8557
Yin S, Tu W, Sheng Y, et al. A highly efficient oxygen evolution catalyst consisting of interconnected nickel-iron-layered double hydroxide and carbon nanodomains. Adv Mater, 2018, 30: 1705106
Yu L, Yang JF, Guan BY, et al. Hierarchical hollow nanoprisms based on ultrathin Ni-Fe layered double hydroxide nanosheets with enhanced electrocatalytic activity towards oxygen evolution. Angew Chem Int Ed, 2018, 57: 172–176
Jia Y, Zhang L, Gao G, et al. A heterostructure coupling of exfoliated Ni-Fe hydroxide nanosheet and defective graphene as a bifunctional electrocatalyst for overall water splitting. Adv Mater, 2017, 29: 1700017
Liu J, Zheng Y, Wang Z, et al. Free-standing single-crystalline NiFe-hydroxide nanoflake arrays: a self-activated and robust electrocatalyst for oxygen evolution. Chem Commun, 2018, 54: 463–466
Gong M, Li Y, Wang H, et al. An advanced Ni–Fe layered double hydroxide electrocatalyst for water oxidation. J Am Chem Soc, 2013, 135: 8452–8455
Chen X, Gao P, Liu H, et al. In situ growth of iron-nickel nitrides on carbon nanotubes with enhanced stability and activity for oxygen evolution reaction. Electrochim Acta, 2018, 267: 8–14
Xu K, Gilles T, Breit B. Asymmetric synthesis of N-allylic indoles via regio-and enantioselective allylation of aryl hydrazines. Nat Commun, 2015, 6: 7616
Chi J, Yu H, Qin B, et al. Vertically aligned FeOOH/NiFe layered double hydroxides electrode for highly efficient oxygen evolution reaction. ACS Appl Mater Interfaces, 2017, 9: 464–471
Tang C, Wang HS, Wang HF, et al. Spatially confined hybridization of nanometer-sized nife hydroxides into nitrogen-doped graphene frameworks leading to superior oxygen evolution reactivity. Adv Mater, 2015, 27: 4516–4522
Stupnišek-Lisac E, Karšulin M. Electrochemical behaviour of nickel in nitric acid. Electrochim Acta, 1984, 29: 1339–1343
McCrory CCL, Jung S, Peters JC, et al. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J Am Chem Soc, 2013, 135: 16977–16987
McCrory CCL, Jung S, Ferrer IM, et al. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J Am Chem Soc, 2015, 137: 4347–4357
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21425103 and 21501192).
Author information
Authors and Affiliations
Corresponding author
Additional information
Hongchao Yang is a PhD candidate in Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese academy of sciences. His current research focuses on electrocatalysis for hydrogen evolution, oxygen reduction/evolution reaction.
Changhong Wang received his BSc degree from Zhengzhou University in 2014. Currently, he is a PhD candidate in Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences under the supervision of Prof. Qiangbin Wang since 2014. His research interest is focused on metal-carbon-based functional materials for electrocatalysis.
Yejun Zhang is a research assistant professor in the Division of Nanobiomedicine at Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences. His current research mainly focuses on the synthesis of semiconductor nanocrystals and their applications in optoelectronics and fluorescence imaging.
Qiangbin Wang is the Director of the Key Laboratory of Nano-Bio Interface, Chinese Academy of Sciences and Professor in nano chemistry at Suzhou Institute of Nano-Tech Nano-Bionics, Chinese Academy of Sciences. One of his research interests concentrates on controlled synthesis and self-assembly of inorganic nanocrystals and their applications in optoelectronics and catalysis.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yang, H., Wang, C., Zhang, Y. et al. Green synthesis of NiFe LDH/Ni foam at room temperature for highly efficient electrocatalytic oxygen evolution reaction. Sci. China Mater. 62, 681–689 (2019). https://doi.org/10.1007/s40843-018-9356-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-018-9356-1