Abstract
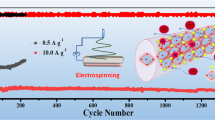
Niobium pentoxide (Nb2O5) has been extensively studied as anode materials for lithium ion batteries (LIBs) due to its good rate performance and safety advantages. However, the intrinsic low electronic conductivity has largely restricted its practical application. In this work, we report the construction of mesoporous T-Nb2O5 nanofibers by electrospinning followed by heat treatment in air. The interconnected mesoporous structure ensures a high surface area with easy electrolyte penetration. When used as anodes for LIBs, the mesoporous Nb2O5 electrode delivers a high reversible specific capacity of 238 mA h g−1 after 1,000 cycles at a current density of 1 A g−1 within a voltage range of 0.01–3.0 V. Even at a higher discharge cut-off voltage window of 1.0–3.0 V, it still possesses a high reversible capacity of 166 mA h g−1 after 200 cycles. Moreover, the porous Nb2O5 electrode also exhibits excellent rate capability. The enhanced electrochemical performances are attributed to the synergistic effects of porous nanofiber structure and unique crystal structure of T-Nb2O5, which has endowed this material a large electrode-electrolyte contact area with improved electronic conductivity.
摘要
五氧化二铌Nb2O5由于其良好的倍率性能和安全性, 作为锂离子电池负极材料被广泛研究. 但是其固有的低电子电导率在很大程度上限制了其电化学性能的发挥. 在本论文中, 我们通过静电纺丝和后续空气热处理构建了具有连续介孔结构的T-Nb2O5纳米纤维. 介孔结构彼此互连, 确保高表面积的同时也促进了电解液的渗透. 当用作锂离子电池负极时在电压窗口为0.01–3.0 V, 电流密度为1 A g−1的条件下, 循环1000圈后可逆比容量达到238 mA h g−1. 即使提高电压窗口到1.0–3.0 V, 在循环200圈后仍然有166 mA h g−1的可逆比容量. 此外, 电极材料还表现出优异的倍率性能. 多孔纳米纤维结构和T-Nb2O5独特晶体结构的协同效应, 增大了电极和电解质的接触面积, 改善了电子传导性, 从而使电化学性能得到提高.
Similar content being viewed by others
References
Bruce PG, Scrosati B, Tarascon JM. Nanomaterials for rechargeable lithium batteries. Angew Chem Int Ed, 2008, 47: 2930–2946
Hu L, Qu B, Li C, et al. Facile synthesis of uniform mesoporous ZnCo2O4 microspheres as a high-performance anode material for Li-ion batteries. J Mater Chem A, 2013, 1: 5596–5602
Wu HB, Chen JS, Hng HH, et al. Nanostructured metal oxide-based materials as advanced anodes for lithium-ion batteries. Nanoscale, 2012, 4: 2526–2542
Ji L, Lin Z, Alcoutlabi M, et al. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ Sci, 2011, 4: 2682–2699
Rahman MM, Rani RA, Sadek AZ, et al. A vein-like nanoporous network of Nb2O5 with a higher lithium intercalation discharge cut-off voltage. J Mater Chem A, 2013, 1: 11019–11025
Lei K, Li F, Mu C, et al. High K-storage performance based on the synergy of dipotassium terephthalate and ether-based electrolytes. Energy Environ Sci, 2017, 10: 552–557
Kong X, Zhu T, Cheng F, et al. Uniform MnCo2O4 porous dumbbells for lithium-ion batteries and oxygen evolution reactions. ACS Appl Mater Interfaces, 2018, 10: 8730–8738
Wang Y, Zhu T, Zhang Y, et al. Rational design of multi-shelled CoO/Co9S8 hollow microspheres for high-performance hybrid supercapacitors. J Mater Chem A, 2017, 5: 18448–18456
Tang C, Liu Y, Xu C, et al. Ultrafine nickel-nanoparticle-enabled SiO2 hierarchical hollow spheres for high-performance lithium storage. Adv Funct Mater, 2018, 28: 1704561–1704568
Park CM, Kim JH, Kim H, et al. Li-alloy based anode materials for Li secondary batteries. Chem Soc Rev, 2010, 39: 3115–3141
Du N, Zhang H, Chen B, et al. Synthesis of polycrystalline SnO2 nanotubes on carbon nanotube template for anode material of lithium-ion battery. Mater Res Bull, 2009, 44: 211–215
Wu ZS, Ren W, Wen L, et al. Graphene anchored with Co3O4 nanoparticles as anode of lithium ion batteries with enhanced reversible capacity and cyclic performance. ACS Nano, 2010, 4: 3187–3194
Zhang J, Ni S, Tang J, et al. The preparation of NiO/C-Ni composite as binder free anode for lithium ion batteries. Mater Lett, 2016, 176: 21–24
Xu H, Wang W. Template synthesis of multishelled Cu2O hollow spheres with a single-crystalline shell wall. Angew Chem Int Ed, 2007, 46: 1489–1492
Arunkumar P, Ashish AG, Babu B, et al. Nb2O5/graphene nanocomposites for electrochemical energy storage. RSC Adv, 2015, 5: 59997–60004
Liu Z, Guan D, Yu Q, et al. Monodisperse and homogeneous SiOx/C microspheres: A promising high-capacity and durable anode material for lithium-ion batteries. Energy Storage Mater, 2018, 13: 112–118
Rani RA, Zoolfakar AS, O’Mullane AP, et al. Thin films and nanostructures of niobium pentoxide: fundamental properties, synthesis methods and applications. J Mater Chem A, 2014, 2: 15683–15703
Schäfer H, Gruehn R, Schulte F. The modifications of niobium pentoxide. Angew Chem Int Ed, 1966, 5: 40–52
Zhao Y, Zhou X, Ye L, et al. Nanostructured Nb2O5 catalysts. Nano Rev, 2012, 3: 17631–17641
Yan L, Rui X, Chen G, et al. Recent advances in nanostructured Nb-based oxides for electrochemical energy storage. Nanoscale, 2016, 8: 8443–8465
Kumagai N. Thermodynamics and kinetics of lithium intercalation into Nb2O5 electrodes for a 2 V rechargeable lithium battery. J Electrochem Soc, 1999, 146: 3203–3210
Kim H, Lim E, Jo C, et al. Ordered-mesoporous Nb2O5/carbon composite as a sodium insertion material. Nano Energy, 2015, 16: 62–70
Wei M, Qi Z, Ichihara M, et al. Synthesis of single-crystal niobium pentoxide nanobelts. Acta Mater, 2008, 56: 2488–2494
Liu F, Xue D. Fabrication of Nb2O5 nanotrees with controlled branching degrees. Phys Scr, 2010, T139: 014074
Luo H, Wei M, Wei K. Synthesis of Nb2O5 nanosheets and its electrochemical measurements. Mater Chem Phys, 2010, 120: 6–9
Varghese B, Haur SC, Lim CT. Nb2O5 nanowires as efficient electron field emitters. J Phys Chem C, 2008, 112: 10008–10012
Viet AL, Reddy MV, Jose R, et al. Nanostructured Nb2O5 polymorphs by electrospinning for rechargeable lithium batteries. J Phys Chem C, 2010, 114: 664–671
Wu L, Lang J, Wang R, et al. Electrospinning synthesis of mesoporous MnCoNiOx@double-carbon nanofibers for sodium-ion battery anodes with pseudocapacitive behavior and long cycle life. ACS Appl Mater Interfaces, 2016, 8: 34342–34352
Liu X, Liu G, Chen H, et al. Facile synthesis of Nb2O5 nanobelts assembled from nanorods and their applications in lithium ion batteries. J Phys Chem Solids, 2017, 111: 8–11
Niu C, Meng J, Han C, et al. VO2 nanowires assembled into hollow microspheres for high-rate and long-life lithium batteries. Nano Lett, 2014, 14: 2873–2878
Chao D, Xia X, Liu J, et al. A V2O5/conductive-polymer core/shell nanobelt array on three-dimensional graphite foam: a high-rate, ultrastable, and freestanding cathode for lithium-ion batteries. Adv Mater, 2014, 26: 5794–5800
Luo Z, Liu L, Ning J, et al. A microporous covalent-organic framework with abundant accessible carbonyl groups for lithium-ion batteries. Angew Chem Int Ed, 2018, 57: 9443–9446
Yao Y, Xu N, Guan D, et al. Facet-selective deposition of FeOx on α-MoO3 nanobelts for lithium storage. ACS Appl Mater Interfaces, 2017, 9: 39425–39431
Sasidharan M, Gunawardhana N, Yoshio M, et al. Nb2O5 hollow nanospheres as anode material for enhanced performance in lithium ion batteries. Mater Res Bull, 2012, 47: 2161–2164
Sing KSW. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem, 1985, 57: 603–619
Shi C, Xiang K, Zhu Y, et al. Preparation and electrochemical properties of nanocable-like Nb2O5/surface-modified carbon nanotubes composites for anode materials in lithium ion batteries. Electrochim Acta, 2017, 246: 1088–1096
Kodama R, Terada Y, Nakai I, et al. Electrochemical and in situ XAFS-XRD investigation of Nb2O5 for rechargeable lithium batteries. J Electrochem Soc, 2006, 153: A583
Wei M, Wei K, Ichihara M, et al. Nb2O5 nanobelts: A lithium intercalation host with large capacity and high rate capability. Electrochem Commun, 2008, 10: 980–983
Liu M, Yan C, Zhang Y. Fabrication of Nb2O5 nanosheets for highrate lithium ion storage applications. Sci Rep, 2015, 5: 8326–8331
Zhao G, Zhang L, Li C, et al. A practical Li ion battery anode material with high gravimetric/volumetric capacities based on TNb2O5/ graphite composite. Chem Eng J, 2017, 328: 844–852
Huang C, Fu J, Song H, et al. General fabrication of mesoporous Nb2O5 nanobelts for lithium ion battery anodes. RSC Adv, 2016, 6: 90489–90493
Sun H, Xin G, Hu T, et al. High-rate lithiation-induced reactivation of mesoporous hollow spheres for long-lived lithium-ion batteries. Nat Commun, 2014, 5: 4526–4533
Li G, Wang X, Ma X. Nb2O5-carbon core-shell nanocomposite as anode material for lithium ion battery. J Energy Chem, 2013, 22: 357–362
Acknowledgements
The authors are grateful to the financial supports from Natural Science Foundation of Hunan Province in China (2018JJ1036), and the Innovation Program of Central South University (2017CX001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Linzhen Lou is a postgraduate student in Professor Anqiang Pan’s Group and will receive his MSc degree in School of Materials Science and Engineering from Central South University. His current research interest is niobium-based materials for anode of lithium ion battery.
Xiangzhong Kong is a PhD candidate student in School of Materials Science and Engineering from Central South University under the supervision of Professor Anqiang Pan. His research interest is silicon-based anode materials for lithium-ion batteries.
Anqiang Pan is currently a full professor in School of Materials Science and Engineering at Central South University. He worked as visiting students at University of Washington and Pacific Northwest National Laboratory in 2008 and 2009, respectively. Then he worked at Nanyang Technological University as a Research Fellow in 2011. He has published >100 papers in peer-reviewed journals. His current interests are rechargeable batteries, supercapacitors and catalysts.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lou, L., Kong, X., Zhu, T. et al. Facile fabrication of interconnected-mesoporous T-Nb2O5 nanofibers as anodes for lithium-ion batteries. Sci. China Mater. 62, 465–473 (2019). https://doi.org/10.1007/s40843-018-9338-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-018-9338-6